Abstract.
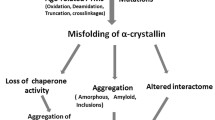
Improper protein folding (misfolding) can lead to the formation of disordered (amorphous) or ordered (amyloid fibril) aggregates. The major lens protein, α-crystallin, is a member of the small heat-shock protein (sHsp) family of intracellular molecular chaperone proteins that prevent protein aggregation. Whilst the chaperone activity of sHsps against amorphously aggregating proteins has been well studied, its action against fibril-forming proteins has received less attention despite the presence of sHsps in deposits found in fibril-associated diseases (e.g. Alzheimer’s and Parkinson’s). In this review, the literature on the interaction of αB-crystallin and other sHsps with fibril-forming proteins is summarized. In particular, the ability of sHsps to prevent fibril formation, their mechanisms of action and the possible in vivo consequences of such associations are discussed. Finally, the fibril-forming propensity of the crystallin proteins and its implications for cataract formation are described along with the potential use of fibrillar crystallin proteins as bionanomaterials.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Additional information
Received 13 June 2008; received after revision 29 July 2008; accepted 05 August 2008
Rights and permissions
About this article
Cite this article
Ecroyd, H., Carver, J.A. Crystallin proteins and amyloid fibrils. Cell. Mol. Life Sci. 66, 62 (2009). https://doi.org/10.1007/s00018-008-8327-4
Published:
DOI: https://doi.org/10.1007/s00018-008-8327-4