Abstract
Introduction
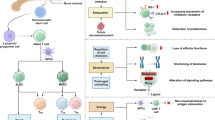
Metabolic reprogramming is one of the important mechanisms of cell differentiation, and different cells have different preferences for energy sources. During the differentiation of naive CD4 + T cells into Th17 and Treg cells, these cells show specific energy metabolism characteristics. Th17 cells depend on enhanced glycolysis, fatty acid synthesis, and glutaminolysis. In contrast, Treg cells are dependent on oxidative phosphorylation, fatty acid oxidation, and amino acid depletion. As a potent antimalarial drug, artesunate has been shown to modulate the Th17/Treg imbalance and regulate cell metabolism.
Methodology
Relevant literatures on ART, cellular metabolism, glycolysis, lipid metabolism, amino acid metabolism, CD4 + T cells, Th17 cells, and Treg cells published from January 1, 2010 to now were searched in PubMed database.
Conclusion
In this review, we will highlight recent advances in which artesunate can restore the Th17/Treg imbalance in disease states by altering T-cell metabolism to influence differentiation and lineage selection. Data from the current study show that few studies have focused on the effect of ART on cellular metabolism. ART can affect the metabolic characteristics of T cells (glycolysis, lipid metabolism, and amino acid metabolism) and interfere with their differentiation lineage, thereby regulating the balance of Th17/Treg and alleviating the symptoms of the disease.




Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ABCA1:
-
ATP-binding cassette transporter A1
- ABCG1:
-
ATP-binding cassette transporter G1
- ACC:
-
Acetyl-CoA carboxylase
- AMPK:
-
AMP-activated protein kinase
- APC:
-
Antigen-presenting cells
- ART:
-
Artesunate
- AST:
-
Aspartate aminotransferase
- c-Rel:
-
REL proto-oncogene
- c-Myc:
-
MYC proto-oncogene
- CCND1:
-
Cyclin D1
- CEBPα:
-
CCAAT/enhancer binding protein α
- ERK:
-
Extracellular regulated protein kinases
- FAO:
-
Fatty acid β-oxidation
- FAS:
-
Fatty acid synthesis
- FASN:
-
Fatty acid synthase
- Fas:
-
Fas cell surface death receptor
- Foxp3:
-
Forkhead Box p3
- GLS:
-
Glutaminase
- GLUD:
-
Glutamate dehydrogenase
- Glut1:
-
Glucose transporter-1
- GOT:
-
Aspartate aminotransferase
- GSH:
-
Glutathione
- GST:
-
Glutathione S-transferase
- G-6-P:
-
Glucose 6-phosphate
- HDAC3:
-
Histone deacetylase 3
- HIF1-α:
-
Hypoxia-inducible factor-1α
- Hk2:
-
Hexokinase 2
- HMGCR:
-
3-Hydroxy-3-methylglutaryl-CoA reductase
- ICER:
-
Inducible cAMP Early Repressor
- IFNγ:
-
Interferon gamma
- IL-17:
-
Interleukin 17
- IL-23R:
-
Interleukin 23 receptor
- IRF4:
-
Interferon regulatory factor 4
- iTreg:
-
Induced T cells
- JAK-STAT:
-
Janus kinase/signal transducer and activator of transcription
- LDHA:
-
Lactate dehydrogenase A
- LDLR:
-
Low-density lipoprotein receptor
- LKB1:
-
Liver kinase B1
- LXRs:
-
Liver X receptors
- MAPK:
-
Mitogen-activated protein kinase
- mTORC1/2:
-
Mammalian target of rapamycin complex 1/2
- nTreg:
-
Natural regulatory T cells
- PHD3:
-
Prolyl hydroxylase 3
- PI3K/Akt:
-
Phosphatidylinositol 3-kinase/protein kinase B
- PKM2:
-
Pyruvate kinase isoform 2
- PPARs:
-
Peroxisome proliferator-activated receptors
- PPARγ:
-
Peroxisome proliferator-activated receptor gamma
- PRMTs:
-
Protein arginine methyltransferases
- PTXF:
-
Pentoxifylline
- Ras:
-
Rat sarcoma
- RORC2:
-
Retinoic acid receptor-associated orphan receptor 2
- ROS:
-
Reactive oxygen species
- SAH:
-
S-adenosylhomocysteine
- SAM:
-
S-adenosylmethionine
- SLC1A5:
-
Solute carrier family 1 member 5
- SLC2A1:
-
Solute carrier family 2 member 1
- SR-BI:
-
Scavenger receptor class B type I
- SREBPs:
-
Sterol response element-binding proteins
- S6K1:
-
Ribosomal protein S6 kinase B1
- TET1/2:
-
Tet methylcytosine dioxygenase 1/2
- Tfh:
-
T follicular helper cells
- Th17:
-
T helper type cells
- Treg:
-
Regulatory T cells
- Tr1:
-
Tregulatory1
- α-ΚG:
-
Alpha-ketoglutaric acid
- 2-DG:
-
2-Deoxyglucose
- 2-HG:
-
2-Hydroxyglutaric acid
- 3-BP:
-
3-Bromopyruvate
References
Faas M, Ipseiz N, Ackermann J, Culemann S, Grüneboom A, Schröder F, et al. IL-33-induced metabolic reprogramming controls the differentiation of alternatively activated macrophages and the resolution of inflammation. Immunity. 2021;54:2531-2546.e5.
Wang H, Yang Y, Liu J, Qian L. Direct cell reprogramming: approaches, mechanisms and progress. Nat Rev Mol Cell Biol. 2021;22:410–24.
Zhang Y, Li F, Chen C, Li Y, Xie W, Huang D, et al. RAGE-mediated T cell metabolic reprogramming shapes T cell inflammatory response after stroke. J Cereb Blood Flow Metab. 2022;42:952–65.
Deng J, Lu C, Zhao Q, Chen K, Ma S, Li Z. The Th17/Treg cell balance: crosstalk among the immune system, bone and microbes in periodontitis. J Periodontal Res. 2022;57:246–55.
Yuan M, Qian X, Huang Y, Ma X, Duan F, Yang Y, et al. Th17 Activation and Th17/Treg imbalance in prolonged anterior intraocular inflammation after ocular alkali burn. Int J Mol Sci. 2022;23:7075.
Wang C, Zhou W, Su G, Hu J, Yang P. Progranulin suppressed autoimmune uveitis and autoimmune neuroinflammation by inhibiting Th1/Th17 cells and promoting Treg cells and M2 macrophages. Neurol Neuroimmunol Neuroinflamm. 2022;9: e1133.
Muik A, Adams HC, Gieseke F, Altintas I, Schoedel KB, Blum JM, et al. DuoBody-CD40x4-1BB induces dendritic-cell maturation and enhances T-cell activation through conditional CD40 and 4–1BB agonist activity. J Immunother Cancer. 2022;10: e004322.
Shenoy AT, De Lyon AC, Arafa EI, Salwig I, Barker KA, Korkmaz FT, et al. Antigen presentation by lung epithelial cells directs CD4+ TRM cell function and regulates barrier immunity. Nat Commun. 2021;12:5834.
Balyan R, Gautam N, Gascoigne NRJ. The ups and downs of metabolism during the lifespan of a T cell. Int J Mol Sci. 2020;21:7972.
Almeida L, Dhillon-LaBrooy A, Carriche G, Berod L, Sparwasser T. CD4+ T-cell differentiation and function: unifying glycolysis, fatty acid oxidation, polyamines NAD mitochondria. J Allergy Clin Immunol. 2021;148:16–32.
Miao Y, Zhang C, Yang L, Zeng X, Hu Y, Xue X, et al. The activation of PPARγ enhances Treg responses through up-regulating CD36/CPT1-mediated fatty acid oxidation and subsequent N-glycan branching of TβRII/IL-2Rα. Cell Commun Signal. 2022;20:48.
Cai F, Jin S, Chen G. The effect of lipid metabolism on CD4+ T cells. Mediators Inflamm. 2021;2021:1–8.
Raud B, Roy DG, Divakaruni AS, Tarasenko TN, Franke R, Ma EH, et al. Etomoxir actions on regulatory and memory T cells are independent of Cpt1a-mediated fatty acid oxidation. Cell Metab. 2018;28:504-515.e7.
Liu S, Liu Z, Shan Z, Liu Y, Chen T, Fang L, et al. Skewed Th17/Treg balance during progression and malignant transformation of oral submucous fibrosis. Oral Dis. 2022;28:2119–30.
Ma Y, Liu C, Xi G, Guan Y, Tang Y, Zhang J, et al. Bioinformatic analysis and cellular assays identify substance P influencing Th17/Treg differentiation via the MyD88 pathway as a potential contributor to the progression of asthma and allergic rhinitis. Dis Markers. 2022;2022:1–11.
Huang F, Wong P, Li J, Lv Z, Xu L, Zhu G, et al. Osteoimmunology: The correlation between osteoclasts and the Th17/Treg balance in osteoporosis. J Cell Mol Med. 2022;26:3591–7.
Zhang S, Gang X, Yang S, Cui M, Sun L, Li Z, et al. The alterations in and the role of the Th17/Treg balance in metabolic diseases. Front Immunol. 2021. https://doi.org/10.3389/fimmu.2021.678355.
Ma N, Zhang Z, Liao F, Jiang T, Tu Y. The birth of artemisinin. Pharmacol Ther. 2020;216: 107658.
Feng X, Cao S, Qiu F, Zhang B. Traditional application and modern pharmacological research of Artemisia annua L. Pharmacol Ther. 2020;216: 107650.
Tu Y. Artemisinin-A gift from traditional Chinese medicine to the world (nobel lecture). Angew Chem Int Ed. 2016;55:10210–26.
Efferth T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin Cancer Biol. 2017;46:65–83.
Efferth T, Oesch F. The immunosuppressive activity of artemisinin-type drugs towards inflammatory and autoimmune diseases. Med Res Rev. 2021;41:3023–61.
Cheong DHJ, Tan DWS, Wong FWS, Tran T. Anti-malarial drug, artemisinin and its derivatives for the treatment of respiratory diseases. Pharmacol Res. 2020;158: 104901.
Bai X-Y, Liu P, Chai Y-W, Wang Y, Ren S-H, Li Y-Y, et al. Artesunate attenuates 2, 4-dinitrochlorobenzene-induced atopic dermatitis by down-regulating Th17 cell responses in BALB/c mice. Eur J Pharmacol. 2020;874: 173020.
Liu J, Hong X, Lin D, Luo X, Zhu M, Mo H. Artesunate influences Th17/Treg lymphocyte balance by modulating Treg apoptosis and Th17 proliferation in a murine model of rheumatoid arthritis. Exp Ther Med. 2017;13:2267–73.
Xiao F, Rui K, Han M, Zou L, Huang E, Tian J, et al. Artesunate suppresses Th17 response via inhibiting IRF4-mediated glycolysis and ameliorates Sjog̈ren’s syndrome. Signal Transduct Target Ther. 2022;7:274.
Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–89.
Ruterbusch M, Pruner KB, Shehata L, Pepper M. In vivo CD4 + T cell differentiation and function: revisiting the Th1/Th2 paradigm. Annu Rev Immunol. 2020;38:705–25.
Maggi L, Santarlasci V, Capone M, Peired A, Frosali F, Crome SQ, et al. CD161 is a marker of all human IL-17-producing T-cell subsets and is induced by RORC. Eur J Immunol. 2010;40:2174–81.
Crome SQ, Wang AY, Kang CY, Levings MK. The role of retinoic acid‐related orphan receptor variant 2 and IL‐17 in the development and function of human CD4 &plus. T cells Eur J Immunol. 2009;39:1480–93.
Mazzoni A, Santarlasci V, Maggi L, Capone M, Rossi MC, Querci V, et al. Demethylation of the RORC2 and IL17A in human CD4+ T lymphocytes defines Th17 origin of nonclassic Th1 cells. J Immunol. 2015;194:3116–26.
Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17–producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32.
Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517.
Loos J, Schmaul S, Noll TM, Paterka M, Schillner M, Löffel JT, et al. Functional characteristics of Th1, Th17, and ex-Th17 cells in EAE revealed by intravital two-photon microscopy. J Neuroinflammation. 2020;17:357.
Millier MJ, Fanning NC, Frampton C, Stamp LK, Hessian PA. Plasma interleukin-23 and circulating IL-17A+IFNγ+ ex-Th17 cells predict opposing outcomes of anti-TNF therapy in rheumatoid arthritis. Arthritis Res Ther. 2022;24:57.
Jiang C, Wang H, Xue M, Lin L, Wang J, Cai G, et al. Reprograming of peripheral Foxp3+ regulatory T cell towards Th17-like cell in patients with active systemic lupus erythematosus. Clin Immunol. 2019;209: 108267.
Shen X, Zhang H, Xie H, Chen L, Li S, Zheng J, et al. Reduced CCR6+IL-17A+Treg cells in blood and CCR6-dependent accumulation of IL-17A+Treg cells in lungs of patients with allergic asthma. Front Immunol. 2021. https://doi.org/10.3389/fimmu.2021.710750.
Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64.
Schuster M, Plaza-Sirvent C, Visekruna A, Huehn J, Schmitz I. Generation of Foxp3+CD25− regulatory T-cell precursors requires c-Rel and IκBNS. Front Immunol. 2019. https://doi.org/10.3389/fimmu.2019.01583.
Ohkura N, Sakaguchi S. Transcriptional and epigenetic basis of Treg cell development and function: its genetic anomalies or variations in autoimmune diseases. Cell Res. 2020;30:465–74.
Zhou W-J, Yang H-L, Shao J, Mei J, Chang K-K, Zhu R, et al. Anti-inflammatory cytokines in endometriosis. Cell Mol Life Sci. 2019;76:2111–32.
Laurence A, Amarnath S, Mariotti J, Kim YC, Foley J, Eckhaus M, et al. STAT3 transcription factor promotes instability of nTreg cells and limits generation of iTreg cells during acute murine graft-versus-host disease. Immunity. 2012;37:209–22.
Dong Y, Yang C, Pan F. Post-translational regulations of Foxp3 in Treg cells and their therapeutic applications. Front Immunol. 2021. https://doi.org/10.3389/fimmu.2021.626172.
Barbi J, Pardoll D, Pan F. Treg functional stability and its responsiveness to the microenvironment. Immunol Rev. 2014;259:115–39.
Muñoz-Rojas AR, Mathis D. Tissue regulatory T cells: regulatory chameleons. Nat Rev Immunol. 2021;21:597–611.
Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, et al. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–53.
Song X, Sun X, Oh SF, Wu M, Zhang Y, Zheng W, et al. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature. 2020;577:410–5.
Yan Y, Ramanan D, Rozenberg M, McGovern K, Rastelli D, Vijaykumar B, et al. Interleukin-6 produced by enteric neurons regulates the number and phenotype of microbe-responsive regulatory T cells in the gut. Immunity. 2021;54:499-513.e5.
Cottrez F, Groux H. Specialization in tolerance: innate CD4+CD25+ versus acquired TR1 and TH3 regulatory T cells. 1. Transplantation. 2004;77:S12–5.
Vences-Catalán F, Rajapaksa R, Srivastava MK, Marabelle A, Kuo C-C, Levy R, et al. Tetraspanin CD81 promotes tumor growth and metastasis by modulating the functions of T regulatory and myeloid-derived suppressor cells. Cancer Res. 2015;75:4517–26.
Cook L, Stahl M, Han X, Nazli A, MacDonald KN, Wong MQ, et al. Suppressive and gut-reparative functions of human type 1 T regulatory cells. Gastroenterology. 2019;157:1584–98.
Ryall JG, Cliff T, Dalton S, Sartorelli V. Metabolic reprogramming of stem cell epigenetics. Cell Stem Cell. 2015;17:651–62.
Almeida L, Lochner M, Berod L, Sparwasser T. Metabolic pathways in T cell activation and lineage differentiation. Semin Immunol. 2016;28:514–24.
Lunt SY, van der Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–64.
Fernie AR, Carrari F, Sweetlove LJ. Respiratory metabolism: glycolysis, the TCA cycle and mitochondrial electron transport. Curr Opin Plant Biol. 2004;7:254–61.
Peek CB, Levine DC, Cedernaes J, Taguchi A, Kobayashi Y, Tsai SJ, et al. Circadian clock interaction with HIF1α mediates oxygenic metabolism and anaerobic glycolysis in skeletal muscle. Cell Metab. 2017;25:86–92.
Nicoli F, Paul S, Appay V. Harnessing the induction of CD8+ T-cell responses through metabolic regulation by pathogen-recognition-receptor triggering in antigen presenting cells. Front Immunol. 2018;9:2372.
Chopp LB, Gopalan V, Ciucci T, Ruchinskas A, Rae Z, Lagarde M, et al. An integrated epigenomic and transcriptomic map of mouse and human αβ T Cell development. Immunity. 2020;53:1182-1201.e8.
Martínez-Méndez D, Mendoza L, Villarreal C, Huerta L. Continuous modeling of T CD4 lymphocyte activation and function. Front Immunol. 2021;12:4424.
Cluxton D, Petrasca A, Moran B, Fletcher JM. Differential regulation of human Treg and Th17 cells by fatty acid synthesis and glycolysis. Front Immunol. 2019;10:115.
Daneshmandi S, Cassel T, Higashi RM, Fan TW-M, Seth P. Phosphogluconate dehydrogenase (6PGD), a key checkpoint in reprogramming of regulatory T cells metabolism and function. Elife. 2021;10:e67476.
Sukonina V, Ma H, Zhang W, Bartesaghi S, Subhash S, Heglind M, et al. FOXK1 and FOXK2 regulate aerobic glycolysis. Nature. 2019;566:279–83.
Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–303.
Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–93.
Huang H, Long L, Zhou P, Chapman NM, Chi H. mTOR signaling at the crossroads of environmental signals and T-cell fate decisions. Immunol Rev. 2020;295:15–38.
Szwed A, Kim E, Jacinto E. Regulation and metabolic functions of mTORC1 and mTORC2. Physiol Rev. 2021;101:1371–426.
Wu S, Zou M-H. AMPK, mitochondrial function, and cardiovascular disease. Int J Mol Sci. 2020;21:4987.
Yang Q, Liu X, Liu Q, Guan Z, Luo J, Cao G, et al. Roles of mTORC1 and mTORC2 in controlling γδ T1 and γδ T17 differentiation and function. Cell Death Differ. 2020;27:2248–62.
Pandit M, Timilshina M, Chang J-H. LKB1-PTEN axis controls Th1 and Th17 cell differentiation via regulating mTORC1. J Mol Med. 2021;99:1139–50.
Siska PJ, van der Windt GJW, Kishton RJ, Cohen S, Eisner W, MacIver NJ, et al. Suppression of Glut1 and glucose metabolism by decreased Akt/mTORC1 signaling drives T cell impairment in B cell leukemia. J Immunol. 2016;197:2532–40.
Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733.
Gilmore TD, Kalaitzidis D, Liang M-C, Starczynowski DT. The c-Rel transcription factor and B-cell proliferation: a deal with the devil. Oncogene. 2004;23:2275–86.
Jeltsch KM, Hu D, Brenner S, Zöller J, Heinz GA, Nagel D, et al. Cleavage of roquin and regnase-1 by the paracaspase MALT1 releases their cooperatively repressed targets to promote TH17 differentiation. Nat Immunol. 2014;15:1079–89.
Okano T, Saegusa J, Nishimura K, Takahashi S, Sendo S, Ueda Y, et al. 3-bromopyruvate ameliorate autoimmune arthritis by modulating Th17/Treg cell differentiation and suppressing dendritic cell activation. Sci Rep. 2017;7:42412.
Fang Y, Shen Z-Y, Zhan Y-Z, Feng X-C, Chen K-L, Li Y-S, et al. CD36 inhibits β-catenin/c-myc-mediated glycolysis through ubiquitination of GPC4 to repress colorectal tumorigenesis. Nat Commun. 2019;10:3981.
Jing Z, Liu Q, He X, Jia Z, Xu Z, Yang B, et al. NCAPD3 enhances Warburg effect through c-myc and E2F1 and promotes the occurrence and progression of colorectal cancer. J Exp Clin Cancer Res. 2022;41:198.
Pacheco-Velázquez SC, Robledo-Cadena DX, Hernández-Reséndiz I, Gallardo-Pérez JC, Moreno-Sánchez R, Rodríguez-Enríquez S. Energy metabolism drugs block triple negative breast metastatic cancer cell phenotype. Mol Pharm. 2018;15:2151–64.
Yang W, Zheng Y, Xia Y, Ji H, Chen X, Guo F, et al. ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat Cell Biol. 2012;14:1295–304.
Liu M, Zhang Z, Wang H, Chen X, Jin C. Activation of AMPK by metformin promotes renal cancer cell proliferation under glucose deprivation through its interaction with PKM2. Int J Biol Sci. 2019;15:617–27.
Layman AAK, Deng G, O’Leary CE, Tadros S, Thomas RM, Dybas JM, et al. Ndfip1 restricts mTORC1 signalling and glycolysis in regulatory T cells to prevent autoinflammatory disease. Nat Commun. 2017;8:15677.
Wei J, Long L, Yang K, Guy C, Shrestha S, Chen Z, et al. Autophagy enforces functional integrity of regulatory T cells by coupling environmental cues and metabolic homeostasis. Nat Immunol. 2016;17:277–85.
Kato H, Perl A. Blockade of Treg cell differentiation and function by the interleukin-21-mechanistic target of rapamycin axis via suppression of autophagy in patients with systemic lupus erythematosus. Arthritis Rheumatol. 2018;70:427–38.
Zeng H, Yang K, Cloer C, Neale G, Vogel P, Chi H. mTORC1 couples immune signals and metabolic programming to establish Treg-cell function. Nature. 2013;499:485–90.
Feng J, Dai W, Mao Y, Wu L, Li J, Chen K, et al. Simvastatin re-sensitizes hepatocellular carcinoma cells to sorafenib by inhibiting HIF-1α/PPAR-γ/PKM2-mediated glycolysis. J Exp Clin Cancer Res. 2020;39:24.
Maturu P, Jones D, Ruteshouser EC, Hu Q, Reynolds JM, Hicks J, et al. Role of cyclooxygenase-2 pathway in creating an immunosuppressive microenvironment and in initiation and progression of Wilms’ tumor. Neoplasia. 2017;19:237–49.
Patterson DG, Kania AK, Price MJ, Rose JR, Scharer CD, Boss JM. An IRF4–MYC–mTORC1 integrated pathway controls cell growth and the proliferative capacity of activated B cells during B cell differentiation in vivo. J Immunol. 2021;207:1798–811.
Mahnke J, Schumacher V, Ahrens S, Käding N, Feldhoff LM, Huber M, et al. Interferon regulatory factor 4 controls TH1 cell effector function and metabolism. Sci Rep. 2016;6:35521.
Bae S, Park PSU, Lee Y, Mun SH, Giannopoulou E, Fujii T, et al. MYC-mediated early glycolysis negatively regulates proinflammatory responses by controlling IRF4 in inflammatory macrophages. Cell Rep. 2021;35: 109264.
Huang SC-C, Smith AM, Everts B, Colonna M, Pearce EL, Schilling JD, et al. Metabolic reprogramming mediated by the mTORC2-IRF4 signaling axis is essential for macrophage alternative activation. Immunity. 2016;45:817–30.
Zhang Y, Wang Y, Li Y, Huang C, Xiao X, Zhong Z, et al. Dihydroartemisinin and artesunate inhibit aerobic glycolysis via suppressing c-Myc signaling in non-small cell lung cancer. Biochem Pharmacol. 2022;198: 114941.
Jin J, Guo D, Wang Y, Jiao W, Li D, He Y. Artesunate inhibits the development of esophageal cancer by targeting HK1 to reduce glycolysis levels in areas with zinc deficiency. Front Oncol. 2022;12:1956.
Berod L, Friedrich C, Nandan A, Freitag J, Hagemann S, Harmrolfs K, et al. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat Med. 2014;20:1327–33.
Miao Y, Wu X, Xue X, Ma X, Yang L, Zeng X, et al. Morin, the PPARγ agonist, inhibits Th17 differentiation by limiting fatty acid synthesis in collagen-induced arthritis. Cell Biol Toxicol. 2022. https://doi.org/10.1007/s10565-022-09769-3.
Yi J, Zhu J, Wu J, Thompson CB, Jiang X. Oncogenic activation of PI3K-AKT-mTOR signaling suppresses ferroptosis via SREBP-mediated lipogenesis. Proc Natl Acad Sci. 2020;117:31189–97.
Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Investig. 2002;109:1125–31.
Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–40.
Brown MS, Goldstein JL. Sterol regulatory element binding proteins (SREBPs): controllers of lipid synthesis and cellular uptake. Nutr Rev. 2009;56:S1-3.
Düvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39:171–83.
Ravacci GR, Brentani MM, Tortelli TC, Torrinhas RSMM, Santos JR, Logullo AF, et al. Docosahexaenoic acid modulates a HER2-associated lipogenic phenotype, induces apoptosis, and increases trastuzumab action in HER2-overexpressing breast carcinoma cells. Biomed Res Int. 2015;2015:1–13.
Yuan M, Pino E, Wu L, Kacergis M, Soukas AA. Identification of Akt-independent regulation of hepatic lipogenesis by mammalian target of rapamycin (mTOR) complex 2. J Biol Chem. 2012;287:29579–88.
Guo Y, Zhao M, Bo T, Ma S, Yuan Z, Chen W, et al. Blocking FSH inhibits hepatic cholesterol biosynthesis and reduces serum cholesterol. Cell Res. 2019;29:151–66.
Mohamed A, Viveiros A, Williams K, de Posse CE. Aβ inhibits SREBP-2 activation through Akt inhibition. J Lipid Res. 2018;59:1–13.
Pallottini V, Guantario B, Martini C, Totta P, Filippi I, Carraro F, et al. Regulation of HMG-CoA reductase expression by hypoxia. J Cell Biochem. 2008;104:701–9.
Gualdoni GA, Mayer KA, Göschl L, Boucheron N, Ellmeier W, Zlabinger GJ. The AMP analog AICAR modulates the T reg /T h 17 axis through enhancement of fatty acid oxidation. FASEB J. 2016;30:3800–9.
Tao W, Cao W, Yu B, Chen H, Gong R, Luorong Q, et al. Hawk tea prevents high-fat diet-induced obesity in mice by activating the AMPK/ACC/SREBP1c signaling pathways and regulating the gut microbiota. Food Funct. 2022;13:6056–71.
Pang Y, Xu X, Xiang X, Li Y, Zhao Z, Li J, et al. High fat activates O-GlcNAcylation and affects AMPK/ACC pathway to regulate lipid metabolism. Nutrients. 2021;13:1740.
Leung KL, Sanchita S, Pham CT, Davis BA, Okhovat M, Ding X, et al. Dynamic changes in chromatin accessibility, altered adipogenic gene expression, and total versus de novo fatty acid synthesis in subcutaneous adipose stem cells of normal-weight polycystic ovary syndrome (PCOS) women during adipogenesis: evidence of cellular programming. Clin Epigenetics. 2020;12:181.
Liu D, Pang Q, Han Q, Shi Q, Zhang Q, Yu H. Wnt10b participates in regulating fatty acid synthesis in the muscle of zebrafish. Cells. 2019;8:1011.
He QJ, Wang P, Liu QQ, Wu QG, Li YF, Wang J, et al. Secreted Wnt6 mediates diabetes-associated centrosome amplification via its receptor FZD4. Am J Physiol Cell Physiol. 2020;318:C48-62.
Nishizuka M, Koyanagi A, Osada S, Imagawa M. Wnt4 and Wnt5a promote adipocyte differentiation. FEBS Lett. 2008;582:3201–5.
Yu W, Chen C-Z, Peng Y, Li Z, Gao Y, Liang S, et al. KRAS affects adipogenic differentiation by regulating autophagy and MAPK activation in 3T3-L1 and C2C12 cells. Int J Mol Sci. 2021;22:13630.
Wang S, Sun L. Silencing Aurora-kinase-A (AURKA) reinforced the sensitivity of diffuse large B-cell lymphoma cells to cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) via suppressing β-Catenin and RAS-extracellular signal-regulated protein kinase (ERK1/2) pathway. Bioengineered. 2021;12:8296–308.
Pan S, Zhang L, Liu Z, Xing H. Myostatin suppresses adipogenic differentiation and lipid accumulation by activating crosstalk between ERK1/2 and PKA signaling pathways in porcine subcutaneous preadipocytes. J Anim Sci. 2021;99:skab287.
Lim SH, Lee HS, Han H-K, Choi C-I. Saikosaponin A and D inhibit adipogenesis via the AMPK and MAPK signaling pathways in 3T3-L1 adipocytes. Int J Mol Sci. 2021;22:11409.
Yadav A, Kataria MA, Saini V, Yadav A. Role of leptin and adiponectin in insulin resistance. Clin Chim Acta. 2013;417:80–4.
Kumar N, Sharma N, Mehan S. Connection between JAK/STAT and PPARγ signaling during the progression of multiple sclerosis: insights into the modulation of T-cells and immune responses in the brain. Curr Mol Pharmacol. 2021;14:823–37.
Yi L, Zhou X, Li T, Liu P, Hai L, Tong L, et al. Notch1 signaling pathway promotes invasion, self-renewal and growth of glioma initiating cells via modulating chemokine system CXCL12/CXCR4. J Exp Clin Cancer Res. 2019;38:339.
Varshney P, Narasimhan A, Mittal S, Malik G, Sardana K, Saini N. Transcriptome profiling unveils the role of cholesterol in IL-17A signaling in psoriasis. Sci Rep. 2016;6:19295.
Sun R, Fang P, Jiang J, Huang C, Wang J, Guo Q, et al. Insulin rescued MCP-1-suppressed cholesterol efflux to large HDL2 particles via ABCA1, ABCG1, SR-BI and PI3K/Akt activation in adipocytes. Cardiovasc Drugs Ther. 2022;36:665–78.
Tsun JGS, Yung S, Chau MKM, Shiu SWM, Chan TM, Tan KCB. Cellular cholesterol transport proteins in diabetic nephropathy. PLoS ONE. 2014;9: e105787.
Kalaany NY, Mangelsdorf DJ. LXRS AND FXR: the Yin and Yang of cholesterol and fat metabolism. Annu Rev Physiol. 2006;68:159–91.
Heller JJ, Qiu J, Zhou L. Nuclear receptors take center stage in Th17 cell–mediated autoimmunity. J Clin Investig. 2011;121:519–21.
Qian Y, Xia L, Wei L, Jiang W. Artesunate attenuates foam cell formation by enhancing cholesterol efflux. Ann Transl Med. 2021;9:1379–1379.
Guccione E, Richard S. The regulation, functions and clinical relevance of arginine methylation. Nat Rev Mol Cell Biol. 2019;20:642–57.
Yuan H, Zhao M, Zhao L, Yun H, Yang G, Geng Y, et al. PRMT5 confers lipid metabolism reprogramming, tumour growth and metastasis depending on the SIRT7-mediated desuccinylation of PRMT5 K387 in tumours. Acta Pharmacol Sin. 2022;43:2373–85.
Webb LM, Sengupta S, Edell C, Piedra-Quintero ZL, Amici SA, Miranda JN, et al. Protein arginine methyltransferase 5 promotes cholesterol biosynthesis–mediated Th17 responses and autoimmunity. J Clin Investig. 2020;130:1683–98.
Lewis BW, Amici SA, Kim H-Y, Shalosky EM, Khan AQ, Walum J, et al. PRMT5 in T cells drives Th17 responses, mixed granulocytic inflammation, and severe allergic airway inflammation. J Immunol. 2022;208:1525–33.
Han C, Ge M, Ho P-C, Zhang L. Fueling T-cell antitumor immunity: amino acid metabolism revisited. Cancer Immunol Res. 2021;9:1373–82.
Johnson MO, Wolf MM, Madden MZ, Andrejeva G, Sugiura A, Contreras DC, et al. Distinct regulation of Th17 and Th1 cell differentiation by glutaminase-dependent metabolism. Cell. 2018;175:1780-1795.e19.
Yu Q, Tu H, Yin X, Peng C, Dou C, Yang W, et al. Targeting glutamine metabolism ameliorates autoimmune hepatitis via inhibiting T cell activation and differentiation. Front Immunol. 2022. https://doi.org/10.3389/fimmu.2022.880262.
Lian G, Gnanaprakasam JR, Wang T, Wu R, Chen X, Liu L, et al. Glutathione de novo synthesis but not recycling process coordinates with glutamine catabolism to control redox homeostasis and directs murine T cell differentiation. Elife. 2018. https://doi.org/10.7554/eLife.36158.
Nakaya M, Xiao Y, Zhou X, Chang J-H, Chang M, Cheng X, et al. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity. 2014;40:692–705.
Amaya ML, Inguva A, Pei S, Jones C, Krug A, Ye H, et al. The STAT3-MYC axis promotes survival of leukemia stem cells by regulating SLC1A5 and oxidative phosphorylation. Blood. 2022;139:584–96.
Spiljar M, Kuchroo VK. Metabolic regulation and function of T helper cells in neuroinflammation. Semin Immunopathol. 2022;44:581–98.
Ren W, Liu G, Yin J, Tan B, Wu G, Bazer FW, et al. Amino-acid transporters in T-cell activation and differentiation. Cell Death Dis. 2017;8:e2655–e2655.
Albrecht J, Zielińska M. Exchange-mode glutamine transport across CNS cell membranes. Neuropharmacology. 2019;161: 107560.
Suzuki S, Venkatesh D, Kanda H, Nakayama A, Hosokawa H, Lee E, et al. GLS2 is a tumor suppressor and a regulator of ferroptosis in hepatocellular carcinoma. Cancer Res. 2022;82:3209–22.
Yang L, Zheng Y, Miao Y, Yan W, Geng Y, Dai Y, et al. Bergenin, a PPARγ agonist, inhibits Th17 differentiation and subsequent neutrophilic asthma by preventing GLS1-dependent glutaminolysis. Acta Pharmacol Sin. 2022;43:963–76.
Xia X, Cao G, Sun G, Zhu L, Tian Y, Song Y, et al. GLS1-mediated glutaminolysis unbridled by MALT1 protease promotes psoriasis pathogenesis. J Clin Investig. 2020;130:5180–96.
Kono M, Yoshida N, Maeda K, Tsokos GC. Transcriptional factor ICER promotes glutaminolysis and the generation of Th17 cells. Proc Natl Acad Sci. 2018;115:2478–83.
Feng M, Xiong G, Cao Z, Yang G, Zheng S, Qiu J, et al. LAT2 regulates glutamine-dependent mTOR activation to promote glycolysis and chemoresistance in pancreatic cancer. J Exp Clin Cancer Res. 2018;37:274.
Zhang X, Wang G, Bi Y, Jiang Z, Wang X. Inhibition of glutaminolysis ameliorates lupus by regulating T and B cell subsets and downregulating the mTOR/P70S6K/4EBP1 and NLRP3/caspase-1/IL-1β pathways in MRL/lpr mice. Int Immunopharmacol. 2022;112: 109133.
Bodineau C, Tomé M, del Murdoch PS, Duran RV. Glutamine, MTOR and autophagy: a multiconnection relationship. Autophagy. 2022;18:2749–50.
Jeon S-W, Conejos JR, Kim J, Kim M-J, Lee J-E, Lee B-S, et al. Supplementing conjugated and non-conjugated L-methionine and acetate alters expression patterns of CSN2, proteins and metabolites related to protein synthesis in bovine mammary cells. J Dairy Res. 2020;87:70–7.
Pettit AP, Jonsson WO, Bargoud AR, Mirek ET, Peelor FF, Wang Y, et al. Dietary methionine restriction regulates liver protein synthesis and gene expression independently of eukaryotic initiation factor 2 phosphorylation in mice. J Nutr. 2017;147:1031–40.
Simile MM, Banni S, Angioni E, Carta G, de Miglio MR, Muroni MR, et al. 5′-Methylthioadenosine administration prevents lipid peroxidation and fibrogenesis induced in rat liver by carbon-tetrachloride intoxication. J Hepatol. 2001;34:386–94.
Panza F, Frisardi V, Capurso C, D’Introno A, Colacicco AM, Vendemiale G, et al. Possible role of S-adenosylmethionine, S-adenosylhomocysteine, and polyunsaturated fatty acids in predementia syndromes and Alzheimer’s disease. J Alzheimer’s Dis. 2009;16:467–70.
Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim S-H, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19:17–30.
Qi L, Martin-Sandoval MS, Merchant S, Gu W, Eckhardt M, Mathews TP, et al. Aspartate availability limits hematopoietic stem cell function during hematopoietic regeneration. Cell Stem Cell. 2021;28:1982-1999.e8.
Weinberg SE, Singer BD, Steinert EM, Martinez CA, Mehta MM, Martínez-Reyes I, et al. Mitochondrial complex III is essential for suppressive function of regulatory T cells. Nature. 2019;565:495–9.
Miao Y, Zheng Y, Geng Y, Yang L, Cao N, Dai Y, et al. The role of GLS1-mediated glutaminolysis/2-HG/H3K4me3 and GSH/ROS signals in Th17 responses counteracted by PPARγ agonists. Theranostics. 2021;11:4531–48.
Funding
This work was supported by the National Nature Science Foundation of China (81860726), the Guangxi Medical and Health Appropriate Technology Development and Promotion Application Project (S2019059).
Author information
Authors and Affiliations
Contributions
Nong had the idea for the article and critically revised the work. Chen performed the literature search and wrote the main manuscript. Tang created Figs. 1, 2, 3, 4 and modified the text format. Chen and Tang have the same contribution to the article, so they are the co-first authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, K., Tang, L. & Nong, X. Artesunate targets cellular metabolism to regulate the Th17/Treg cell balance. Inflamm. Res. 72, 1037–1050 (2023). https://doi.org/10.1007/s00011-023-01729-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-023-01729-9