Abstract.
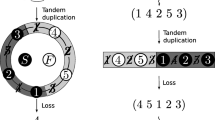
Circular permutations of genes during molecular evolution often are regarded as elusive, although a simple model can explain these rearrangements. The model assumes that first a gene duplication of the precursor gene occurs in such a way that both genes become fused in frame, leading to a tandem protein. After generation of a new start codon within the 5′ part of the tandem gene and a stop at an equivalent position in the 3′ part of the gene, a protein is encoded that represents a perfect circular permutation of the precursor gene product. The model is illustrated here by the molecular evolution of adenine-N6 DNA methyltransferases. β- and γ-type enzymes of this family can be interconverted by a single circular permutation event. Interestingly, tandem proteins, proposed as evolutionary intermediates during circular permutation, can be directly observed in the case of adenine methyltransferases, because some enzymes belonging to type IIS, like the FokI methyltransferase, are built up by two fused enzymes, both of which are active independently of each other. The mechanism for circular permutation illustrated here is very easy and applicable to every protein. Thus, circular permutation can be regarded as a normal process in molecular evolution and a changed order of conserved amino acid motifs should not be interpreted to argue against divergent evolution.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 17 November 1998 / Accepted: 19 February 1999
Rights and permissions
About this article
Cite this article
Jeltsch, A. Circular Permutations in the Molecular Evolution of DNA Methyltransferases. J Mol Evol 49, 161–164 (1999). https://doi.org/10.1007/PL00006529
Issue Date:
DOI: https://doi.org/10.1007/PL00006529