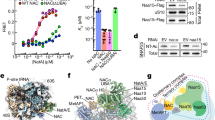
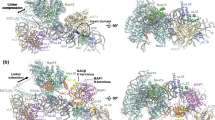
Abstract.
Nascent-polypeptide-associated complex (NAC) is a heterodimeric complex which can reversibly bind to eukaryotic ribosomes. NAC is located in direct proximity to newly synthesized polypeptide chains as they emerge from the ribosome. Although its function is thought to be conserved from yeast to humans our current knowledge about what NAC actually does in a living cell is incomplete. It has been suggested that NAC is a (i) dynamic component of the ribosomal exit tunnel, providing a shield for nascent polypeptides, (ii) negative regulator of translocation into the endoplasmic reticulum and (iii) positive regulator of translocation into the mitochondria. However, none of these hypotheses is generally accepted. Moreover, the individual subunits of NAC have been implicated in processes related to transcription rather than translation, and it is currently under debate whether NAC might be a protein of dual function. This review attempts to summarize the data from different fields and to discuss the partly controversial results in a common context.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
RID="*"
ID="*"Corresponding author.
Rights and permissions
About this article
Cite this article
Rospert, S., Dubaquié, Y. & Gautschi, M. Nascent-polypeptide-associated complex. CMLS, Cell. Mol. Life Sci. 59, 1632–1639 (2002). https://doi.org/10.1007/PL00012490
Issue Date:
DOI: https://doi.org/10.1007/PL00012490