Abstract
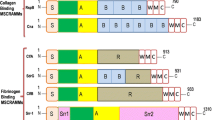
Both Gram-negative and Gram-positive pathogens display a multitude of proteins and protein assemblies (pili or fimbriae) on their cell surfaces, which are often used for adherence and initiate colonization and pathogenesis. Adhesive proteins known as MSCRAMMs (microbial surface components recognizing adhesive matrix molecules), anchored by a specific enzyme called sortase in Gram-positive bacteria, target the host’s extracellular matrix proteins (ECM) like collagen, fibrinogen and fibronectin. In the past decade, structural analysis by X-ray crystallography has enhanced our understanding of the interactions between MSCRAMMs and the host ECM by revealing several novel structural features that dictate surface protein assembly and the mode of their adhesion to host tissue. The latest focus is on the recently discovered Gram-positive bacterial pili, assembly of which is assisted by yet another specific sortase. Novel features like inter- and intra-molecular isopeptide bonds that facilitate the stability of the pilins, and intra-molecular donor strand complementation to stabilize the adhesin-target interactions are specific to Gram-positive bacteria. This chapter describes and discusses the common structural details between surface proteins and pilins of Gram-positive bacteria and biological implications emanating from these structures.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Baker C, Edward M (2001) Group B streptococcal infections. In: Remington JS, Klein JO (eds) Infectious diseases of the fetus and newborn infant. WB Saunders, Philadelphia: PA, pp 1091–1156
Bingham RJ, Rudino-Pinera E, Meenan NA, Schwarz-Linek U, Turkenburg JP, Höök M, Garman EF, Potts JR (2008) Crystal structures of fibronectin-binding sites from Staphylococcus aureus FnBPA in complex with fibronectin domains. Proc Natl Acad Sci USA 105:12254–12258
Bork P, Holm L, Sander C (1994) The immunoglobulin fold. Structural classification, sequence patterns and common core. J Mol Biol 242:309–320
Bowden MG, Heuck AP, Ponnuraj K, Kolosova E, Choe D, Gurusiddappa S, Narayana SV, Johnson AE, Höök M (2008) Evidence for the “dock, lock, and latch” ligand binding mechanism of the staphylococcal microbial surface component recognizing adhesive matrix molecules (MSCRAMM) SdrG. J Biol Chem 283:638–647
Budzik JM, Poor CB, Faull KF, Whitelegge JP, He C, Schneewind O (2009) Intramolecular amide bonds stabilize pili on the surface of bacilli. Proc Natl Acad Sci USA 106:19992–19997
Choudhury D, Thompson A, Stojanoff V, Langermann S, Pinkner J, Hultgren SJ, Knight SD (1999) X-ray structure of the FimC-FimH chaperone-adhesin complex from uropathogenic Escherichia coli. Science 285:1061–1066
Deivanayagam CC, Rich RL, Carson M, Owens RT, Danthuluri S, Bice T, Höök M, Narayana SV (2000) Novel fold and assembly of the repetitive B region of the Staphylococcus aureus collagen-binding surface protein. Structure 8:67–78
Deivanayagam CC, Wann ER, Chen W, Carson M, Rajashankar KR, Höök M, Narayana SV (2002) A novel variant of the immunoglobulin fold in surface adhesins of Staphylococcus aureus: crystal structure of the fibrinogen-binding MSCRAMM, clumping factor A. EMBO J 21: 6660–6672
Dramsi S, Caliot E, Bonne I, Guadagnini S, Prevost MC, Kojadinovic M, Lalioui L, Poyart C, Trieu-Cuot P (2006) Assembly and role of pili in group B streptococci. Mol Microbiol 60:1401–1413
D‘Souza SE, Haas TA, Piotrowicz RS, Byers-Ward V, McGrath DE, Soule HR, Cierniewski C, Plow EF, Smith JW (1994) Ligand and cation binding are dual functions of a discrete segment of the integrin β3 subunit: cation displacement is involved in ligand binding. Cell 79: 659–667
Emsley J, Knight CG, Farndale RW, Barnes MJ (2004) Structure of the integrin α2β1-binding collagen peptide. J Mol Biol 335:1019–1028
Emsley J, Knight CG, Farndale RW, Barnes MJ, Liddington RC (2000) Structural basis of collagen recognition by integrin α2β1. Cell 101:47–56
Flock JI, Froman G, Jonsson K, Guss B, Signas C, Nilsson B, Raucci G, Höök M, Wadstrom T, Lindberg M (1987) Cloning and expression of the gene for a fibronectin-binding protein from Staphylococcus aureus. EMBO J 6:2351–2357
Froman G, Switalski LM, Speziale P, Höök M (1987) Isolation and characterization of a fibronectin receptor from Staphylococcus aureus. J Biol Chem 262:6564–6571
Ganesh VK, Rivera JJ, Smeds E, Ko YP, Bowden MG, Wann ER, Gurusiddappa S, Fitzgerald JR, Höök M (2008) A structural model of the Staphylococcus aureus ClfA-fibrinogen interaction opens new avenues for the design of anti-staphylococcal therapeutics. PLoS Pathog 4:e1000226
Gaspar AH, Ton-That H (2006) Assembly of distinct pilus structures on the surface of Corynebacterium diphtheriae. J Bacteriol 188:1526–1533
Greenberg CS, Birckbichler PJ, Rice RH (1991) Transglutaminases: multifunctional cross-linking enzymes that stabilize tissues. FASEB J 5:3071–3077
Hahn E, Wild P, Hermanns U, Sebbel P, Glockshuber R, Häner M, Taschner N, Burkhard P, Aebi U, Müller SA (2002) Exploring the 3D molecular architecture of Escherichia coli type 1 pili. J Mol Biol 323:845–857
Harpaz Y, Chothia C (1994) Many of the immunoglobulin superfamily domains in cell adhesion molecules and surface receptors belong to a new structural set which is close to that containing variable domains. J Mol Biol 238:528–539
Hawiger J, Timmons S, Strong DD, Cottrell BA, Riley M, Doolittle RF (1982) Identification of a region of human fibrinogen interacting with staphylococcal clumping factor. Biochemistry 21:1407–1413
Herrick S, Blanc-Brude O, Gray A, Laurent G (1999) Fibrinogen. Int J Biochem Cell Biol 31: 741–746
Hilleringmann M, Ringler P, Müller SA, De Angelis G, Rappuoli R, Ferlenghi I, Engel A (2009) Molecular architecture of Streptococcus pneumoniae TIGR4 pili. EMBO J 28:3921–3930
House-Pompeo K, Boles JO, Höök M (1994) Characterization of bacterial adhesin interactions with extracellular matrix components utilizing biosensor technology. Methods: Companion Method Enzymol 6:134–142
Izoré T, Contreras-Martel C, El Mortaji L, Manzano C, Terrasse R, Vernet T, Di Guilmi AM, Dessen A (2010) Structural basis of host cell recognition by the pilus adhesin from Streptococcus pneumoniae. Structure 18:106–115
Jonsson K, Signas C, Müller HP, Lindberg M (1991) Two different genes encode fibronectin binding proteins in Staphylococcus aureus. The complete nucleotide sequence and characterization of the second gene. Eur J Biochem 202:1041–1048
Kang HJ, Baker EN (2009) Intramolecular isopeptide bonds give thermodynamic and proteolytic stability to the major pilin protein of Streptococcus pyogenes. J Biol Chem 284: 20729–20737
Kang HJ, Coulibaly F, Clow F, Proft T, Baker EN (2007) Stabilizing isopeptide bonds revealed in Gram-positive bacterial pilus structure. Science 318:1625–1628
Kang HJ, Paterson NG, Gaspar AH, Ton-That H, Baker EN (2009) The Corynebacterium diphtheriae shaft pilin SpaA is built of tandem Ig-like modules with stabilizing isopeptide and disulfide bonds. Proc Natl Acad Sci USA 106:16967–16971
Kehrel B (1995) Platelet-collagen interactions. Semin Thromb Hemost 21:123–129
Kloczewiak M, Timmons S, Lukas TJ, Hawiger J (1984) Platelet receptor recognition site on human fibrinogen. Synthesis and structure-function relationship of peptides corresponding to the carboxy-terminal segment of the γ chain. Biochemistry 23:1767–1774
Krishnan V, Gaspar AH, Ye N, Mandlik A, Ton-That H, Narayana SV (2007) An IgG-like domain in the minor pilin GBS52 of Streptococcus agalactiae mediates lung epithelial cell adhesion. Structure 15:893–903
Kuusela P (1978) Fibronectin binds to Staphylococcus aureus. Nature 276:718–720
Lauer P, Rinaudo CD, Soriani M, Margarit I, Maione D, Rosini R, Taddei AR, Mora M, Rappuoli R, Grandi G, Telford JL (2005) Genome analysis reveals pili in Group B Streptococcus. Science 309:105
Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S (1980) Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature 284:67–68
Liu Q, Ponnuraj K, Xu Y, Ganesh VK, Sillanpää J, Murray BE, Narayana SV, Höök M (2007) The Enterococcus faecalis MSCRAMM ACE binds its ligand by the collagen hug model. J Biol Chem 282:19629–19637
Mandlik A, Swierczynski A, Das A, Ton-That H (2007) Corynebacterium diphtheriae employs specific minor pilins to target human pharyngeal epithelial cells. Mol Microbiol 64:111–124
Navarre WW, Schneewind O (1999) Surface proteins of Gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol Mol Biol Rev 63:174–229
Patti JM, Allen BL, McGavin MJ, Höök M (1994a) MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol 48:585–617
Patti JM, Boles JO, Höök M (1993) Identification and biochemical characterization of the ligand binding domain of the collagen adhesin from Staphylococcus aureus. Biochemistry 32: 11428–11435
Patti JM, Bremell T, Krajewska-Pietrasik D, Abdelnour A, Tarkowski A, Ryden C, Höök M (1994b) The Staphylococcus aureus collagen adhesin is a virulence determinant in experimental septic arthritis. Infect Immun 62:152–161
Patti JM, House-Pompeo K, Boles JO, Garza N, Gurusiddappa S, Höök M (1995) Critical residues in the ligand-binding site of the Staphylococcus aureus collagen-binding adhesin (MSCRAMM). J Biol Chem 270:12005–12011
Pickart CM (2001) Mechanisms underlying ubiquitination. Annu Rev Biochem 70:503–533
Ponnuraj K, Bowden MG, Davis S, Gurusiddappa S, Moore D, Choe D, Xu Y, Höök M, Narayana SV (2003) A “dock, lock, and latch” structural model for a staphylococcal adhesin binding to fibrinogen. Cell 115:217–228
Ponnuraj K, Narayana SV (2007) Crystal structure of ACE19, the collagen binding subdomain of Enterococus faecalis surface protein ACE. Proteins 69:199–203
Proft T, Baker EN (2009) Pili in Gram-negative and Gram-positive bacteria – structure, assembly and their role in disease. Cell Mol Life Sci 66:613–635
Remaut H, Rose RJ, Hannan TJ, Hultgren SJ, Radford SE, Ashcroft AE, Waksman G (2006) Donor-strand exchange in chaperone-assisted pilus assembly proceeds through a concerted β strand displacement mechanism. Mol Cell 22:831–842
Rich RL, Demeler B, Ashby K, Deivanayagam CC, Petrich JW, Patti JM, Narayana SV, Höök M (1998) Domain structure of the Staphylococcus aureus collagen adhesin. Biochemistry 37:15423–15433
Richardson JS (1981) The anatomy and taxonomy of protein structure. Adv Prot Chem 34:167–399
Rosini R, Rinaudo CD, Soriani M, Lauer P, Mora M, Maione D, Taddei A, Santi I, Ghezzo C, Brettoni C, Buccato S, Margarit I, Grandi G, Telford JL (2006) Identification of novel genomic islands coding for antigenic pilus-like structures in Streptococcus agalactiae. Mol Microbiol 61:126–141
Sauer FG, Knight SD, Waksman Gj, Hultgren SJ (2000a) PapD-like chaperones and pilus biogenesis. Semin Cell Dev Biol 11:27–34
Sauer FG, Mulvey MA, Schilling JD, Martinez JJ, Hultgren SJ (2000b) Bacterial pili: molecular mechanisms of pathogenesis. Curr Opin Microbiol 3:65–72
Schneewind O, Model P, Fischetti VA (1992) Sorting of protein A to the staphylococcal cell wall. Cell 70:267–281
Schwarz-Linek U, Höök M, Potts JR (2006) Fibronectin-binding proteins of Gram-positive cocci. Microbes Infect 8:2291–2298
Schwarz-Linek U, Pilka ES, Pickford AR, Kim JH, Höök M, Campbell ID, Potts JR (2004) High affinity streptococcal binding to human fibronectin requires specific recognition of sequential F1 modules. J Biol Chem 279:39017–39025
Schwarz-Linek U, Werner JM, Pickford AR, Gurusiddappa S, Kim JH, Pilka ES, Briggs JA, Gough TS, Höök M, Campbell ID, Potts JR (2003) Pathogenic bacteria attach to human fibronectin through a tandem β-zipper. Nature 423:177–181
Scott JR, Zahner D (2006) Pili with strong attachments: Gram-positive bacteria do it differently. Mol Microbiol 62:320–330
Shoulders MD, Raines RT (2009) Collagen structure and stability. Annu Rev Biochem 78: 929–958
Smith JW, Piotrowicz RS, Mathis D (1994) A mechanism for divalent cation regulation of β 3-integrins. J Biol Chem 269:960–967
Speziale P, Raucci G, Visai L, Switalski LM, Timpl R, Höök M (1986) Binding of collagen to Staphylococcus aureus Cowan 1. J Bacteriol 167:77–81
Swierczynski A, Ton-That H (2006) Type III pilus of corynebacteria: pilus length is determined by the level of its major pilin subunit. J Bacteriol 188:6318–6325
Symersky J, Patti JM, Carson M, House-Pompeo K, Teale M, Moore D, Jin L, Schneider A, DeLucas LJ, Höök M, Narayana SV (1997) Structure of the collagen-binding domain from a Staphylococcus aureus adhesin. Nat Struct Biol 4:833–838
Telford JL, Barocchi MA, Margarit I, Rappuoli R, Grandi G (2006) Pili in Gram-positive pathogens. Nat Rev Microbiol 4:509–519
Ton-That H, Schneewind O (2003) Assembly of pili on the surface of Corynebacterium diphtheriae. Mol Microbiol 50:1429–1438
Ton-That H, Schneewind O (2004) Assembly of pili in Gram-positive bacteria. Trends Microbiol 12:228–234
Waksman G, Hultgren SJ (2009) Structural biology of the chaperone-usher pathway of pilus biogenesis. Nat Rev Microbiol 7:765–774
Wann ER, Gurusiddappa S, Höök M (2000) The fibronectin-binding MSCRAMM FnbpA of Staphylococcus aureus is a bifunctional protein that also binds to fibrinogen. J Biol Chem 275:13863–13871
Xu Y, Liang X, Chen Y, Koehler TM, Höök M (2004) Identification and biochemical characterization of two novel collagen binding MSCRAMMs of Bacillus anthracis. J Biol Chem 279:51760–51768
Yanagawa R, Otsuki K, Tokui T (1968) Electron microscopy of fine structure of Corynebacterium renale with special reference to pili. Jpn J Vet Res 16:31–37
Zong Y, Xu Y, Liang X, Keene DR, Höök A, Gurusiddappa S, Höök M, Narayana SV (2005) A “collagen hug” model for Staphylococcus aureus CNA binding to collagen. EMBO J 24:4224–4236
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Krishnan, V., Narayana, S.V. (2011). Crystallography of Gram-Positive Bacterial Adhesins. In: Linke, D., Goldman, A. (eds) Bacterial Adhesion. Advances in Experimental Medicine and Biology, vol 715. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-0940-9_11
Download citation
DOI: https://doi.org/10.1007/978-94-007-0940-9_11
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-0939-3
Online ISBN: 978-94-007-0940-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)