Abstract
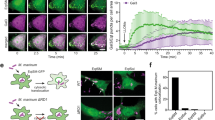
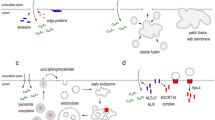
The perforation of the plasmalemma by pore-forming toxins causes an influx of Ca2+ and an efflux of cytoplasmic proteins. In order to ensure cellular survival, lesions have to be identified, plugged and removed from the membrane. The Ca2+-driven fusion of lysosomes with the plasma membrane leads to hydrolysis of sphingomyelin by acid sphingomyelinase and a formation of ceramide platforms in the outer leaflet of the lipid bilayer. We propose that the negative curvature, promoted by tighter packing of lipids in the outer layer, leads to an inward vesiculation of the damaged area for its endocytotic uptake and internal degradation. In contrast, the activation of neutral sphingomyelinase triggers the production of ceramide within the inner leaflet of the lipid bilayer, thereby promoting an outward curvature, which enables the cell to shed the membrane-containing toxin pore into the extracellular space. In this process, ceramide is supported by members of the annexin protein family which act as Ca2+ sensors and as membrane fusion agents.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Abdel Shakor AB, Kwiatkowska K, Sobota A (2004) Cell surface ceramide generation precedes and controls FcgammaRII clustering and phosphorylation in rafts. J Biol Chem 279:36778–36787
Andrieu-Abadie N, Levade T (2002) Sphingomyelin hydrolysis during apoptosis. Biochim Biophys Acta 1585:126–134
Ashida H, Mimuro H, Ogawa M, Kobayashi T, Sanada T, Kim M, Sasakawa C (2011) Host-pathogen interactions: cell death and infection: a double-edged sword for host and pathogen survival. J Cell Biol. doi:10.1083/jcb.201108081:
Babiychuk EB, Monastyrskaya K, Draeger A (2008) Fluorescent annexin A1 reveals dynamics of ceramide platforms in living cells. Traffic 9:1757–1775
Babiychuk EB, Monastyrskaya K, Potez S, Draeger A (2009) Intracellular Ca(2+) operates a switch between repair and lysis of streptolysin O-perforated cells. Cell Death Differ 16:1126–1134
Babiychuk EB, Monastyrskaya K, Potez S, Draeger A (2010) Blebbing confers resistance against cell lysis. Cell Death Differ 18:80–89
Babiychuk EB, Atanassoff AP, Monastyrskaya K, Brandenberger C, Studer D, Allemann C, Draeger A (2011) The targeting of plasmalemmal ceramide to mitochondria during apoptosis. PLoS One 6:e23706
Bezombes C, Grazide S, Garret C, Fabre C, Quillet-Mary A, Muller S, Jaffrezou JP, Laurent G (2004) Rituximab antiproliferative effect in B-lymphoma cells is associated with acid-sphingomyelinase activation in raft microdomains. Blood 104:1166–1173
Bleicken S, Classen M, Padmavathi PV, Ishikawa T, Zeth K, Steinhoff HJ, Bordignon E (2010) Molecular details of Bax activation, oligomerization, and membrane insertion. J Biol Chem 285:6636–6647
Borgonovo B, Cocucci E, Racchetti G, Podini P, Bachi A, Meldolesi J (2002) Regulated exocytosis: a novel, widely expressed system. Nat Cell Biol 4:955–962
Caler EV, Chakrabarti S, Fowler KT, Rao S, Andrews NW (2001) The Exocytosis-regulatory protein synaptotagmin VII mediates cell invasion by Trypanosoma cruzi. J Exp Med 193:1097–1104
Chamberlain LH, Burgoyne RD, Gould GW (2001) SNARE proteins are highly enriched in lipid rafts in PC12 cells: implications for the spatial control of exocytosis. Proc Natl Acad Sci USA 98:5619–5624
Chambers R (1917) Microdissection studies. I. The visible structure of cell protoplasm and death changes. Am J Physiol 43:1–12
Chernomordik LV, Kozlov MM (2008) Mechanics of membrane fusion. Nat Struct Mol Biol 15:675–683
Draeger A, Monastyrskaya K, Babiychuk EB (2011) Plasma membrane repair and cellular damage control: the annexin survival kit. Biochem Pharmacol 81:703–712
Fernandes MC, Cortez M, Flannery AR, Tam C, Mortara RA, Andrews NW (2011) Trypanosoma cruzi subverts the sphingomyelinase-mediated plasma membrane repair pathway for cell invasion. J Exp Med 208:909–921
Fine M, Llaguno MC, Lariccia V, Lin MJ, Yaradanakul A, Hilgemann DW (2011) Massive endocytosis driven by lipidic forces originating in the outer plasmalemmal monolayer: a new approach to membrane recycling and lipid domains. J Gen Physiol 137:137–154
Freche B, Reig N, van der Goot FG (2007) The role of the inflammasome in cellular responses to toxins and bacterial effectors. Semin Immunopathol 29:249–260
Gil C, Cubi R, Blasi J, Aguilera J (2006) Synaptic proteins associate with a sub-set of lipid rafts when isolated from nerve endings at physiological temperature. Biochem Biophys Res Commun 348:1334–1342
Goni FM, Alonso A (2009) Effects of ceramide and other simple sphingolipids on membrane lateral structure. Biochim Biophys Acta 1788:169–177
Grassme H, Jekle A, Riehle A, Schwarz H, Berger J, Sandhoff K, Kolesnick R, Gulbins E (2001) CD95 signaling via ceramide-rich membrane rafts. J Biol Chem 276:20589–20596
Grassme H, Jendrossek V, Bock J, Riehle A, Gulbins E (2002) Ceramide-rich membrane rafts mediate CD40 clustering. J Immunol 168:298–307
Grassme H, Riethmuller J, Gulbins E (2007) Biological aspects of ceramide-enriched membrane domains. Prog Lipid Res 46:161–170
Gulbins E, Kolesnick R (2003) Raft ceramide in molecular medicine. Oncogene 22:7070–7077
Gulbins E, Li PL (2006) Physiological and pathophysiological aspects of ceramide. Am J Physiol Regul Integr Comp Physiol 290:R11–R26
Hannun YA, Obeid LM (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9:139–150
Hilgemann DW, Fine M (2011) Mechanistic analysis of massive endocytosis in relation to functionally defined surface membrane domains. J Gen Physiol 137:155–172
Holopainen JM, Angelova MI, Kinnunen PK (2000) Vectorial budding of vesicles by asymmetrical enzymatic formation of ceramide in giant liposomes. Biophys J 78:830–838
Idone V, Tam C, Goss JW, Toomre D, Pypaert M, Andrews NW (2008) Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J Cell Biol 180:905–914
Janmey PA, Kinnunen PK (2006) Biophysical properties of lipids and dynamic membranes. Trends Cell Biol 16:538–546
Keyel PA, Heid ME, Salter RD (2011) Macrophage responses to bacterial toxins: a balance between activation and suppression. Immunol Res 50:118–123
Kirschnek S, Paris F, Weller M, Grassme H, Ferlinz K, Riehle A, Fuks Z, Kolesnick R, Gulbins E (2000) CD95-mediated apoptosis in vivo involves acid sphingomyelinase. J Biol Chem 275:27316–27323
Kolesnick RN, Goni FM, Alonso A (2000) Compartmentalization of ceramide signaling: physical foundations and biological effects. J Cell Physiol 184:285–300
Lariccia V, Fine M, Magi S, Lin MJ, Yaradanakul A, Llaguno MC, Hilgemann DW (2011) Massive calcium-activated endocytosis without involvement of classical endocytic proteins. J Gen Physiol 137:111–132
Lewis RS (2003) Calcium oscillations in T-cells: mechanisms and consequences for gene expression. Biochem Soc Trans 31:925–929
Li R, Blanchette-Mackie EJ, Ladisch S (1999) Induction of endocytic vesicles by exogenous C(6)-ceramide. J Biol Chem 274:21121–21127
Lopez-Montero I, Rodriguez N, Cribier S, Pohl A, Velez M, Devaux PF (2005) Rapid transbilayer movement of ceramides in phospholipid vesicles and in human erythrocytes. J Biol Chem 280:25811–25819
Lorusso A, Covino C, Priori G, Bachi A, Meldolesi J, Chieregatti E (2006) Annexin2 coating the surface of enlargeosomes is needed for their regulated exocytosis. EMBO J 25:5443–5456
McNeil PL, Kirchhausen T (2005) An emergency response team for membrane repair. Nat Rev Mol Cell Biol 6:499–505
McNeil PL, Steinhardt RA (2003) Plasma membrane disruption: repair, prevention, adaptation. Annu Rev Cell Dev Biol 19:697–731
McNeil AK, Rescher U, Gerke V, McNeil PL (2006) Requirement for annexin A1 in plasma membrane repair. J Biol Chem 281:35202–35207
Morgan BP (1989) Complement membrane attack on nucleated cells: resistance, recovery and non-lethal effects. Biochem J 264:1–14
Morgan BP, Luzio JP, Campbell AK (1986) Intracellular Ca2+ and cell injury: a paradoxical role of Ca2+ in complement membrane attack. Cell Calcium 7:399–411
Morgan BP, Dankert JR, Esser AF (1987) Recovery of human neutrophils from complement attack: removal of the membrane attack complex by endocytosis and exocytosis. J Immunol 138:246–253
Ninomiya Y, Kishimoto T, Miyashita Y, Kasai H (1996) Ca2+-dependent exocytotic pathways in Chinese hamster ovary fibroblasts revealed by a caged-Ca2+ compound. J Biol Chem 271:17751–17754
Paris F, Grassme H, Cremesti A, Zager J, Fong Y, Haimovitz-Friedman A, Fuks Z, Gulbins E, Kolesnick R (2001) Natural ceramide reverses Fas resistance of acid sphingomyelinase(−/−) hepatocytes. J Biol Chem 276:8297–8305
Pedrosa Ribeiro CMP, McKay RR, Hosoki E, Bird GS, Putney JW (2000) Effects of elevated cytoplasmic calcium and protein kinase C on endoplasmic reticulum structure and function in HEK293 cells. Cell Calcium 27:175–185
Perrotta C, Bizzozero L, Cazzato D, Morlacchi S, Assi E, Simbari F, Zhang Y, Gulbins E, Bassi MT, Rosa P, Clementi E (2010) Syntaxin 4 is required for acid sphingomyelinase activity and apoptotic function. J Biol Chem 285:40240–40251
Pilzer D, Gasser O, Moskovich O, Schifferli JA, Fishelson Z (2005) Emission of membrane vesicles: roles in complement resistance, immunity and cancer. Springer Semin Immunopathol 27:375–387
Potez S, Luginbuhl M, Monastyrskaya K, Hostettler A, Draeger A, Babiychuk EB (2011) Tailored protection against plasmalemmal injury by annexins with different Ca2+ sensitivities. J Biol Chem 286:17982–17991
Salaun C, Gould GW, Chamberlain LH (2005) Lipid raft association of SNARE proteins regulates exocytosis in PC12 cells. J Biol Chem 280:19449–19453
Siskind LJ, Kolesnick RN, Colombini M (2002) Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. J Biol Chem 277:26796–26803
Szabadkai G, Rizzuto R (2004) Participation of endoplasmic reticulum and mitochondrial calcium handling in apoptosis: more than just neighborhood? FEBS Lett 567:111–115
Tam C, Idone V, Devlin C, Fernandes MC, Flannery A, He X, Schuchman E, Tabas I, Andrews NW (2010) Exocytosis of acid sphingomyelinase by wounded cells promotes endocytosis and plasma membrane repair. J Cell Biol 189:1027–1038
Tang N, Ong WY, Zhang EM, Chen P, Yeo JF (2007) Differential effects of ceramide species on exocytosis in rat PC12 cells. Exp Brain Res 183:241–247
Tepper AD, Ruurs P, Wiedmer T, Sims PJ, Borst J, van Blitterswijk WJ (2000) Sphingomyelin hydrolysis to ceramide during the execution phase of apoptosis results from phospholipid scrambling and alters cell-surface morphology. J Cell Biol 150:155–164
Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M (2008) Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319:1244–1247
van Blitterswijk WJ, van der Luit AH, Veldman RJ, Verheij M, Borst J (2003) Ceramide: second messenger or modulator of membrane structure and dynamics? Biochem J 369:199–211
Walev I, Tappe D, Gulbins E, Bhakdi S (2000) Streptolysin O-permeabilized granulocytes shed L-selectin concomitantly with ceramide generation via neutral sphingomyelinase. J Leukoc Biol 68:865–872
Walev I, Bhakdi SC, Hofmann F, Djonder N, Valeva A, Aktories K, Bhakdi S (2001) Delivery of proteins into living cells by reversible membrane permeabilization with streptolysin-O. Proc Natl Acad Sci USA 98:3185–3190
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Wien
About this chapter
Cite this chapter
Draeger, A., Babiychuk, E.B. (2013). Ceramide in Plasma Membrane Repair. In: Gulbins, E., Petrache, I. (eds) Sphingolipids in Disease. Handbook of Experimental Pharmacology, vol 216. Springer, Vienna. https://doi.org/10.1007/978-3-7091-1511-4_17
Download citation
DOI: https://doi.org/10.1007/978-3-7091-1511-4_17
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-1510-7
Online ISBN: 978-3-7091-1511-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)