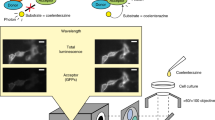
Abstract
Knowledge of how and when proteins interact in living cells is fundamental to our understanding of cellular biology, and bioluminescence resonance energy transfer (BRET) provides an increasingly popular mechanism for studying these interactions in real time. The technique utilises heterologously expressed fusion proteins linking a bioluminescent donor or complementary acceptor fluorophore to proteins of interest. Resonance energy transfer between these fusion proteins is then detected when they are in close proximity, indicative of association either directly or as part of a complex. BRET is particularly useful for real-time monitoring of ligand-modulated interactions as dynamic changes in protein complex assembly can be observed in a live cell environment.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Pfleger, K. D. G., and Eidne, K. A. (2006) Illuminating insights into protein-protein interactions using bioluminescence resonance energy transfer (BRET). Nat Methods 3, 165–174.
Pfleger, K. D. G., Seeber, R. M., and Eidne, K. A. (2006) Bioluminescence resonance energy transfer (BRET) for the real-time detection of protein–protein interactions. Nat Protoc 1 , 337–345.
Milligan, G., and Bouvier, M. (2005) Methods to monitor the quaternary structure of G-protein-coupled receptors. FEBS J 272, 2914–2925.
Wu, P., and Brand, L. (1994) Resonance energy transfer: methods and applications. Anal Biochem 218 , 1–13.
Pfleger, K. D. G., and Eidne, K. A. (2003) New technologies: bioluminescence resonance energy transfer (BRET) for the detection of real time interactions involving G-protein coupled receptors. Pituitary 6 , 141–151.
Pfleger, K. D. G., and Eidne, K. A. (2005) Monitoring the formation of dynamic G protein-coupled receptor–protein complexes in living cells. Biochem J 385 , 625–637.
De, A., Loening, A. M., and Gambhir, S. S. (2007) An improved bioluminescence resonance energy transfer strategy for imaging intracellular events in single cells and living subjects. Cancer Res 67 , 7175–7183.
Nagai, T., Ibata, K., Park, E. S., Kubota, M., Mikoshiba, K., and Miyawaki, A. (2002) A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol 20, 87–90.
Pfleger, K. D. G., Dromey, J. R., Dalrymple, M. B., Lim, E. M. L., Thomas, W. G., and Eidne, K. A. (2006) Extended bioluminescence resonance energy transfer (eBRET) for monitoring prolonged protein–protein interactions in live cells. Cell Signal 18 , 1664–1670.
Kocan, M., See, H.B., Seeber, R. M., Eidne, K. A., and Pfleger, K. D. G. (2008) Demonstration of improvements to the bioluminescence resonance energy transfer (BRET) technology for the mointoring of G protein-coupled receptors in live cells. J Biomol Screen 13, 888–898.
Hamdan, F. F., Audet, M., Garneau, P., Pelletier, J., and Bouvier, M. (2005) High-throughput screening of G protein-coupled receptor antagonists using a bioluminescence resonance energy transfer 1-based beta-arrestin2 recruitment assay. J Biomol Screen 10 , 463–475.
Mercier, J. F., Salahpour, A., Angers, S., Breit, A., and Bouvier, M. (2002) Quantitative assessment of β1- and β2-adrenergic receptor homo- and heterodimerization by bioluminescence resonance energy transfer. J Biol Chem 277 , 44925–44931.
Germain-Desprez, D., Bazinet, M., Bouvier, M., and Aubry, M. (2003) Oligomerization of transcriptional intermediary factor 1 regulators and interaction with ZNF74 nuclear matrix protein revealed by bioluminescence resonance energy transfer in living cells. J Biol Chem 278 , 22367–22373.
Breit, A., Lagace, M., and Bouvier, M. (2004) Hetero-oligomerization between β2- and β3-adrenergic receptors generates a β-adrenergic signalling unit with distinct functional properties. J Biol Chem 279, 28756–28765.
Kroeger, K. M., Hanyaloglu, A. C., Seeber, R. M., Miles, L. E., and Eidne, K. A. (2001) Constitutive and agonist-dependent homo-oligomerization of the thyrotropin-releasing hormone receptor. Detection in living cells using bioluminescence resonance energy transfer. J Biol Chem 276 , 12736–12743.
Ayoub, M. A., Couturier, C., Lucas-Meunier, E., Angers, S., Fossier, P., Bouvier, M., and Jockers, R. (2002) Monitoring of ligand-independent dimerization and ligand-induced conformational changes of melatonin receptors in living cells by bioluminescence resonance energy transfer. J Biol Chem 277 , 21522–21528.
Zhang, J. -H., Chung, T. D. Y., and Oldenburg, K. R. (1999) A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Bio Mol Screen 4 , 67–73.
Angers, S., Salahpour, A., Joly, E., Hilairet, S., Chelsky, D., Dennis, M., and Bouvier, M. (2000) Detection of β2-adrenergic receptor dimerization in living cells using bioluminescence resonance energy transfer (BRET). Proc Natl Acad Sci USA 97, 3684–3689.
McVey, M., Ramsay, D., Kellett, E., Rees, S., Wilson, S., Pope, A. J., and Milligan, G. (2001) Monitoring receptor oligomerization using time-resolved fluorescence resonance energy transfer and bioluminescence resonance energy transfer. J Biol Chem 276, 14092–14099.
James, J. R., Oliveira, M. I., Carmo, A. M., Iaboni, A., and Davis, S. J. (2006) A rigorous experimental framework for detecting protein oligomerization using bioluminescence resonance energy transfer. Nat Methods 3 , 1001–1006.
Bouvier, M., Heveker, N., Jockers, R., Marullo, S., and Milligan, G. (2007) BRET analysis of GPCR oligomerization: newer does not mean better. Nat Methods 4 , 3–4.
Salahpour, A., and Masri, B. (2007) Experimental challenge to a ‘rigorous’ BRET analysis of GPCR oligomerization. Nat Methods 4 , 599–600.
Acknowledgements
The author would like to thank Professor Sanjiv Sam Gambhir and Dr. Atsushi Miyawaki for generously providing Rluc8 and Venus cDNA, respectively. The author and his work using the BRET methodology have been funded by the National Health and Medical Research Council of Australia in the form of a Peter Doherty Research Fellowship (#353709), Project Grants (#404087 and #566736) and a Development Grant (#513780).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2009 Humana Press, a part of Springer Science+Business Media, LLC
About this protocol
Cite this protocol
Pfleger, K.D. (2009). Analysis of Protein–Protein Interactions Using Bioluminescence Resonance Energy Transfer. In: Rich, P., Douillet, C. (eds) Bioluminescence. Methods in Molecular Biology™, vol 574. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-60327-321-3_14
Download citation
DOI: https://doi.org/10.1007/978-1-60327-321-3_14
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-60327-320-6
Online ISBN: 978-1-60327-321-3
eBook Packages: Springer Protocols