Abstract
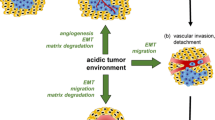
The tumor microenvironment is characterized by hypoxia, acidosis as well as other metabolic and biochemical alterations. Its role in cancer progression is increasingly appreciated especially on invasive capacity and the formation of metastasis. The effect of acidosis on metastasis formation of two rat carcinoma cell lines was studied in the animal model. In order to analyze the pH dependency of different steps of metastasis formation, invasiveness, cell adhesion and migration of AT-1 prostate cancer cells as well as possible underlying cell signaling pathways were studied in vitro.
Acidosis significantly increased the formation of lung metastases of both tumor cell lines in vivo. In vitro, extracellular acidosis neither enhanced invasiveness nor affected cell adhesion to a plastic or to an endothelial layer. However, cellular motility was markedly elevated at pH 6.6 and this effect was sustained even when extracellular pH was switched back to pH 7.4. When analyzing the underlying mechanism, a prominent role of ROS in the induction of migration was observed. Signaling through the MAP kinases ERK1/2 and p38 as well as Src family kinases was not involved. Thus, cancer cells in an acidic microenvironment can acquire enhanced motility, which is sustained even if the tumor cells leave their acidic microenvironment e.g. by entering the blood stream. This increase depended on elevated ROS production and may contribute to the augmented formation of metastases of acidosis-primed tumor cells in vivo.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Vaupel P, Kallinowski F, Okunieff P (1989) Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res 49:6449–6465
Gerweck LE, Seetharaman K (1996) Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res 56:1194–1198
Gatenby RA, Gawlinski ET, Gmitro AF et al (2006) Acid-mediated tumor invasion: a multidisciplinary study. Cancer Res 66:5216–5223
Giusti I, D’Ascenzo S, Millimaggi D et al (2008) Cathepsin B mediates the pH-dependent proinvasive activity of tumor-shed microvesicles. Neoplasia 10:481–488
Moellering RE, Black KC, Krishnamurty C et al (2008) Acid treatment of melanoma cells selects for invasive phenotypes. Clin Exp Metastasis 25:411–425
Rofstad EK, Mathiesen B, Kindem K, Galappathi K (2006) Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res 66:6699–6707
Workman P, Aboagye EO, Balkwill F et al (2010) Guidelines for the welfare and use of animals in cancer research. Br J Cancer 102:1555–1577
Riemann A, Schneider B, Ihling A et al (2011) Acidic environment leads to ROS-induced MAPK signaling in cancer cells. PLoS One 6:e22445
Porporato PE, Payen VL, Pérez-Escuredo J et al (2014) A mitochondrial switch promotes tumor metastasis. Cell Rep 8:754–766
Sauvant C, Nowak M, Wirth C et al (2008) Acidosis induces multi-drug resistance in rat prostate cancer cells (AT1) in vitro and in vivo by increasing the activity of the p-glycoprotein via activation of p38. Int J Cancer 123:2532–2542
Estrella V, Chen T, Lloyd M et al (2013) Acidity generated by the tumor microenvironment drives local invasion. Cancer Res 73:1524–1535
Robey IF, Baggett BK, Kirkpatrick ND et al (2009) Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res 69:2260–2268
Acknowledgments
This study was supported by the Deutsche Krebshilfe (Grants 106774/106906), the BMBF (ProNet-T3 Ta-04) and the Wilhelm-Roux program of the Medical School, Universität Halle-Wittenberg.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media, New York
About this paper
Cite this paper
Riemann, A., Schneider, B., Gündel, D., Stock, C., Gekle, M., Thews, O. (2016). Acidosis Promotes Metastasis Formation by Enhancing Tumor Cell Motility. In: Elwell, C.E., Leung, T.S., Harrison, D.K. (eds) Oxygen Transport to Tissue XXXVII. Advances in Experimental Medicine and Biology, vol 876. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-3023-4_27
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3023-4_27
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-3022-7
Online ISBN: 978-1-4939-3023-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)