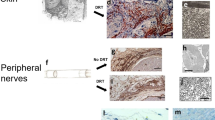
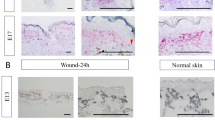
Abstract
Mammalian fetal skin regenerates perfectly, but adult skin repairs by the formation of scar tissue. The cause of this imperfect repair by adult skin is not understood. In contrast, wounded adult amphibian (urodeles and anurans) skin is like mammalian fetal skin in that it repairs by regeneration, not scarring. Scar-free wound repair in adult Xenopus is associated with expression of the paired homeobox transcription factor Prx1 by mesenchymal cells of the wound, a feature shared by mesenchymal cells of the regeneration blastema of the axolotl limb. Furthermore, mesenchymal cells of Xenopus skin wounds that harbor the mouse Prx1-limb-enhancer as a transgene exhibit activation of the enhancer despite the fact that they are Xenopus cells, suggesting that the mouse Prx1 enhancer possesses all elements required for its activation in skin wound healing, even though activation of the same enhancer in the mouse is not seen in the wounded skin of an adult mouse. Elucidation of the role of the Prx1 gene in amphibian skin wound healing will help to clarify the molecular mechanisms of scarless wound healing. Shifting the molecular mechanism of wound repair in mammals to that of amphibians, including reactivation of the Prx1-limb-enhancer, will be an important clue to stimulate scarless wound repair in mammalian adult skin. Finding or creating Prx1-positive stem cells in adult mammal skin by activating the Prx1-limb-enhancer may be a fast and reliable way to provide for scarless skin wound repair, and even directly lead to limb regeneration in mammals.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- AEC:
-
Apical epithelial cap
- D:
-
Dermis
- E:
-
Epidermis
- ECM:
-
Extracellular matrix
- EPC:
-
Endothelial progenitor cell
- H:
-
Hypodermis
- MIF:
-
Migration inhibitory factor
- MMP:
-
Matrix metalloproteinase
- PDGF:
-
Platelet-derived growth factor
- TGF:
-
Transforming growth factor
- VEGF:
-
Vascular endothelial growth factor
References
Abe R, Donnelly SC, Peng T, Bucala R, Metz CN (2001) Peripheral blood fibrocytes: differntiation pathway and migration to wound sites. J Immunol 166:7556–7562
Bellingan GJ, Caldwell H, Howie SE, Dransfield I, Haslett C (1996) In vivo fate of the inflammatory macrophage during the resolution of inflammation: inflammatory macrophages do not die locally, but emigrate to the draining lymph nodes. J Immunol 157:2577–2585
Bodemer CW (1959) Observations on the mechanism of induction of supernumerary limbs in adult Triturus viridescens. J Exp Zool 140:79–99
Carlson MR, Bryant SV, Gardiner DM (1998) Expression of Msx-2 during development, regeneration, and wound healing in axolotl limbs. J Exp Zool 282:715–723
Cass DL, Sylvester KG, Yang EY, Crombleholme TM, Adzick NS (1997) Myofibroblast persistence in fetal sheep wounds is associated with scar formation. J Pediatr Surg 32:1017–1021
Cass DL, Bullard KM, Sylvester KG, Yang EY, Sheppard D, Herlyn M, Adzick NS (1998) Epidermal integrin expression is upregulated rapidly in human fetal wound repair. J Pediatr Surg 33:312–316
Ceradini DJ, Kulkami AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC (2004) Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 10:858–864
Chen WY, Grant ME, Schor AM, Schor SL (1989) Differences between adult and foetal fibroblasts in the regulation of hyaluronate synthesis: correlation with migratory activity. J Cell Sci 94:577–584
Clark RAF (1996) The molecular and cellular biology of wound repair, 2nd edn. Plenum Press, New York, pp 427–474
Cowin AJ, Brosnan MP, Holmes TM, Ferguson MW (1998) Endogenous inflammatory response to dermal wound healing in the fetal and adult mouse. Dev Dyn 212:385–393
Cowin AJ, Holmes TM, Brosnan P, Ferguson MW (2001) Expression of TGF-beta and its receptors in murine fetal and adult dermal wounds. Eur J Dermatol 11:424–431
Dent JN (1962) Limb regeneration in larvae and metamorphosing individuals of the South African clawed toad. J Morphol 110:61–77
Ellis IR, Schor SL (1996) Differential effects of TGF-beta1 on hyaluronan synthesis by fetal and adult skin fibroblasts: implications for cell migration and wound healing. Exp Cell Res 228:326–333
Endo T, Bryant SV, Gardiner DM (2004) A stepwise model system for limb regeneration. Dev Biol 270:135–145
Enoch S, Grey JE, Harding KG (2006) Recent advances and emerging treatments. Br Med J 332:962–965
Ferguson MW, O’Kane S (2004) Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Philos Trans R Soc Lond B Biol Sci 359:839–850
Gordillo GM, Sen CK (2003) Revisiting the essential role of oxygen in wound healing. Am J Surg 186:259–263
Grieb G, Piatkowski A, Simons D, Hormann N, Dewor M, Steffans G, Bernhagen J, Pallua N (2010) Macrophage migration inhibitory factor is a potential inducer of endothelial progenitor cell mobilization after flap operation. Surgery 151:268–277
Hopkinson-Woolley J, Hughes D, Gordon S, Martin P (1994) Macrophage recruitment during limb development and wound healing in the embryonic and foetal mouse. J Cell Sci 107:1159–1167
Levenson SM, Geever EF, Vrowley LV, Oates JF 3rd, Berard CW, Rosen H (1965) The healing of rat skin wounds. Ann Surg 161:293–308
Lévesque M, Villiard E, Roy S (2010) Skin wound healing in axolotls: a scarless process. J Exp Zool B Mol Dev Evol 314:684–697
Lheureux E (1977) Importance of limb tissue associations in the development of nerve-induced supernumerary limbs in the newt Pleurodeles waltlii Michah. J Embryol Exp Morphol 38:151–173
Lorenz HP, Adzick NS (1993) Scarless skin wound repair in the fetus. West J Med 159:350–355
Malcolm M, Holder N (1984) Axial characteristics of nerve induced supernumerary limbs in the axolotl. Roux’s Arch.\ Dev Biol 193:394–401
Martin P, Lewis J (1992) Actin cables and epidermal movement in embryonic wound healing. Nature 360:179–183
Martin P, Dickson MC, Millan FA, Akhurst RJ (1993) Rapid induction and clearance of TGF beta 1 is an early response to wounding in the mouse embryo. Dev Genet 14:225–238
Martin P, D’Souza D, Martin J, Grose R, Cooper L, Maki R, McKercher SR (2003) Wound healing in the PU.1 null mouse–tissue repair is not dependent on inflammatory cells. Curr Biol 13:1122–1128
Mast BA (1992) The skin. In: Cohen IK, Diegelmann RF, Lindblad WJ (eds) Wound Healing: Biochemical and Clinical Aspects. WB Saunders, Philadelphia, pp 344–355
Matoltsy AG, Downes AM, Sweeney TM (1968) Studies of the epidermal water barrier. II investigation of the chemical nature of the water barrier. J Invest Dermatol 50:19–26
McKean DM, Sisbarro L, Ilic D, Kaplan-Alburquerque N, Nemenoff R, Weiser-Evans M, Kern MJ, Jones PL (2003) FAK induces expression of Prx1 to promote tenascin-C-dependent fibroblast migration. J Cell Biol 161:393–402
Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ (2002) Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell 109:693–705
Muneoka K, Holler-Dinsmore G, Bryant SV (1986) Intrinsic control of regenerative loss in Xenopus laevis limbs. J Exp Zool 240:47–54
Muneoka K, Sassoon D (1992) Molecular aspects of regeneration in developing vertebrate limbs. Dev Biol 152:37–49
Nickoloff BJ, Mitra RS, Riser BL, Dixit VM, Varani J (1988) Modulation of keratinocyte motility. Correlation with production of extracellular matrix molecules in response to growth promoting and antiproliferative factors. Am J Pathol 132:543–551
Oztürk S, Deveci M, Sengezer M, Günhan O (2001) Results of artificial inflammation in scarless foetal wound healing: an experimental study in foetal lambs. Br J Plast Surg 54:47–52
Poll CP (2009) Wound Management in Amphibians: etiology and Treatment of Cutaneous Lesions. J Exotic Pet Med 18:20–35
Putnins EE, Firth JD, Lohachitranont A, Uitto VJ, Larjava H (1999) Keratinocyte growth factor (KGF) promotes keratinocyte cell attachment and migration on collagen and fibronectin. Cell Adhes Commun 7:211–221
Repesh LA, Oberpriller JC (1978) Scanning electron microscopy of epidermal cell migration in wound healing during limb regeneration in the adult newt, Notophthalmus viridescens. Am J Anat 151:539–555
Reynolds S, Holder N, Fernandes M (1983) The form and structure of supernumerary hindlimbs formed following skin grafting and nerve deviation in the newt Triturus cristatus. J Embryol Exp Morphol 77:221–241
Satoh A, Gardiner DM, Bryant SV, Endo T (2007) Nerve-induced ectopic limb blastemas in the axolotl are equivalent to amputation-induced blastemas. Dev Biol 312:231–244
Seifert AW, Monaghan JR, Voss SR, Maden M (2012) Skin regeneration in adult axolotls: a blueprint for scar-free healing in vertebrates. PLoS ONE 7(4):e32875
Sessions SK, Bryant SV (1988) Evidence that regenerative ability is an intrinsic property of limb cells in Xenopus. J Exp Zool 247:39–44
Shah M, Foreman DM, Ferguson MW (1994) Neutralising antibody to TGF-beta 1,2 reduces cutaneous scarring in adult rodents. J Cell Sci 107:1137–1157
Shah M, Foreman DM, Ferguson MW (1995) Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci 108:985–1002
Soo C, Beanes SR, Hu FY, Zhang X, Dang C, Chang G, Wang Y, Nishimura I, Freymiller E, Longaker MT, Lorenz HP, Ting K (2003) Ontogenetic transition in fetal wound transforming growth factor-beta regulation correlates with collagen organization. Am J Pathol 163:2459–2476
Stocum DL (2011) The role of peripheral nerves in urodele limb regeneration. Eur J Neurosci 34:908–916
Suzuki M, Satoh A, Ide H, Tamura K (2005) Nerve-dependent and -independent events in blastema formation during Xenopus froglet limb regeneration. Dev Biol 286:361–375
Suzuki M, Yakushiji N, Nakada Y, Satoh A, Ide H, Tamura K (2006) Limb regeneration in Xenopus laevis froglet. Sci World J 6:26–37
Suzuki M, Satoh A, Ide H, Tamura K (2007) Transgenic Xenopus with prx1 limb enhancer reveals crucial contribution of MEK/ERK and PI3 K/AKT pathways in blastema formation during limb regeneration. Dev Biol 304:675–686
Tamura K, Ohgo S, Yokoyama H (2010) Limb blastema cell: a stem cell for morphological regeneration. Dev Growth Differ 52:89–99
Tepper OM, Capla JM, Galiano RD, Ceradini DJ, Callaghan MJ, Kleinman ME, Gurtner GC (2005) Adult vasculogenesis occurs through in situ recruitment, proliferatioon, and tubulization of circulating bone marrow-derived cells. Blood 105:1068–1077
Ulrich D, Lichtenegger F, Unglaub F, Smeets R, Pallua N (2005) Effect of chronic wound exudates and MMP-2/-9 inhibitor on angiogenesis in vitro. Plast Reconstr Surg 116:539–545
Velazquez OC (2007) Angiogenesis and vasculogenesis: inducing the growth of new blood vessels and wound healing by stimulation of bone marrow-derived progenitor cell mobilization and homing. J Vasc Surg 45:A39–A47
Werner S, Richard G (2003) Regulation of wound healing by growth factors and cytokines. Physiol Rev 83:835–870
Whitby DJ, Ferguson MW (1991) The extracellular matrix of lip wounds in fetal, neonatal and adult mice. Development 122:651–668
Whitby DJ, Longaker MT, Harrison MR, Adzick NS, Ferguson MW (1991) Rapid epithelialisation of fetal wounds is associated with the early deposition of tenascin. J Cell Sci 99:583–586
Wong VW, Sorkin M, Glotzbach JP, Longaker MT, Gurtner GC (2011) Surgical approaches to create murine models of human wound healing. J Biomed Biotechnol 969618. Epub 1 Dec 2010
Wu L, Siddiqui A, Morris DE, Cox DA, Roth SI, Mustoe TA (1977) Transforming growth factor beta 3 (TGF beta 3) accelerates wound healing without alteration of scar prominence. Histologic and competitive reverse-transcription-polymerase chain reaction studies. Arch Surg 132:753–760
Wulff BC, Parent AE, Meleski MA, DiPietro LA, Schrementi ME, Wilgus TA (2012) Mast cells contribute to scar formation during fetal wound healing. J Invest Dermatol 132:458–465
Yang EV, Bryant SV (1994) Developmental regulation of a matrix metalloproteinase during regeneration of axolotl appendages. Dev Biol 166:696–703
Yakushiji N, Yokoyama H, Tamura K (2009) Repatterning in amphibian limb regeneration: a model for study of genetic and epigenetic control of organ regeneration. Semin Cell Dev Biol 20:565–574
Yokoyama H, Maruoka T, Aruga A, Amano T, Ohgo S, Shiroishi T, Tamura K (2011) Prx-1 expression in Xenopus laevis scarless skin-wound healing and its resemblance to epimorphic regeneration. J Invest Dermatol 131:2477–2485
Yoshizato K (2007) Molecular mechanism and evolutional significance of epithelial-mesenchymal interactions in the body- and tail-dependent metamorphic transformation of anuran larval skin. Int Rev Cytol 260:213–260
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Kawasumi, A., Sagawa, N., Hayashi, S., Yokoyama, H., Tamura, K. (2012). Wound Healing in Mammals and Amphibians: Toward Limb Regeneration in Mammals. In: Heber-Katz, E., Stocum, D. (eds) New Perspectives in Regeneration. Current Topics in Microbiology and Immunology, vol 367. Springer, Berlin, Heidelberg. https://doi.org/10.1007/82_2012_305
Download citation
DOI: https://doi.org/10.1007/82_2012_305
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-35809-8
Online ISBN: 978-3-642-35810-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)