Abstract
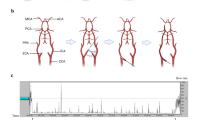
The reliable and reproducible creation of an animal model of focal cerebral ischemia is not easily accomplished. Using a transortibal approach, we showed that occlusion of the posterior cerebral artery (PCA), middle cerebral artery (MCA), and the contralateral anterior cerebral artery (ACA) created a large cortical and subcortical stroke in the non-human primate (NHP). Subsequently, we created the same stroke endovascularly in the NHP. Using the endovascular stroke model in the NHP, we measured brain temperature with thermocouples and cerebral blood flow (CBF) by stable xenon CT in one NHP, and CMRO2 and CBF by positron emission tomography (PET) in another NHP.
Two female non-human primates (M mulatto) weighing 7.0 and 8.0 kg, respectively, were studied under fentanyl-diazepam anesthesia with continuous monitoring of arterial blood pressure, rectal temperature, and end-tidal CO2 with intermittent blood gas measurements. Using an endovascular approach, the PCA (P2), MCA (Ml), and the ICA at the bifurcation and contralateral ACA produced a large hemispheric stroke. In the right ischemic hemisphere, temperatures increased by 2°C–3°C. PET measurement of CBF and CMRO2 showed that CMRO2 increased in the region of the ischemic stroke. We found that both hyperthermia and hypermetabolism occur in acute stroke.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
A. Tamura, D. I. Graham, J. McCulloch, and G. M. Teasdale, Focal cerebral ischemia in the rat. I. Description of technique and early neuropathological consequences following middle cerebral artery occlusion, J. Cereb. Blood Flow Metab. 1, 53–60 (1981).
K. Minematsu, L. Li, M. Fisher, C. H. Sotak, M. A. Davis, and M. S. Fiandaca, Diffusion-weighted magnetic resonance imaging: rapid and quantitative detection of focal brain ischemia, Neurol. 42(1), 235–240 (1992).
M. Sakoh, L. Ostergaard, L. Rohl, D. F. Smith, C. Z. Simonsen, J. C. Sorensen, P. V. Poulsen, C. Gyldensted, S. Sakaki, and A. Gjedde, Relationship between residual cerebral blood flow and oxygen metabolism as predictive of ischemic tissue viability: sequential multitracer positron emission tomography scanning of middle cerebral artery occlusion during the critical first 6 hours after stroke in pigs, J. Neurosurg. 93(4), 647–657 (2000).
J. G. Frazee, X. Luo, G. Luan, D. S. Hinton, D. A. Hovda, M. S. Shiroishi, and L. T. Barcliff, Retrograde transvenous neuroperfusion: a back door treatment for stroke, Stroke 29(9), 1912–1916 (1998).
J. Huang, J. Mocco, T. F. Choudhri, A. Poisik, S. J. Popilskis, R. Emerson, R. L. DelaPaz, A. G. Khandji, D. J. Pinsky, and E. S. Connolly Jr., A modified transorbital baboon model of reperfused stroke, Stroke 31(12), 3054–3063 (2000).
M. Horowitz, A. Kassam, E. M. Nemoto, C. A. Jungreis, G. R. Rao, and J. Arimoto, An endovascular primate model for the production of a middle cerebral artery ischemic infarction, Intervent. Radiol. 7, 223–228 (2001).
S. Ohta, E. Meyer, H. Fujita, D. C. Reutens, A. Evans, and A. Gjedde, Cerebral [15O]water clearance in humans determined by PET: I. Theory and normal values, J. Cereb. Blood Flow Metab. 16, 765–780 (1966).
H. Iida, I. Kanno, S. Miura, M. Murakami, K. Takahashi, and K. Uemura, Error analysis of a quantitative cerebral blood flow measurement using H2 l5Oautoradiography and positron emission tomography with respect to the dispersion of the input function, J. Cereb. Blood Flow Metab. 6, 536–545 (1986).
M. A. Mintun, M. E. Raichle, W. R. Martin, and P. Herscovitch, Brain oxygen utilization measured with O-15 radiotracers and positron emission tomography, J. Nucl. Med. 25, 177–187 (1983).
S. Ohta, E. Meyer, and C. J. Thompson, Oxygen consumption of the living human brain measured after a single inhalation of positron emitting oxygen, J. Cereb. Blood Flow Metab. 12, 179–192 (1992).
R. Busto, W. D. Dietrich, M. Y. T. Globus, I. Valdes, P. Schienberg, and M. D. Ginsberg, Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury, J. Cereb. Blood Flow Metab. 7, 729–738 (1987).
R. F. Albrecht 2nd, C. T. Wass, and W. L. Lanier, Occurrence of potentially detrimental temperature alterations in hospitalized patients at risk for brain injury, Mayo Clin. Proc. 73(7), 629–635 (1988).
J. Castillo, A. Davalos, J. Marrugat, and M. Noya, Timing for fever-related brain damage in acute ischemic stroke, Stroke 29, 2455–2460 (1998).
M. D. Ginsburg, and R. Busto, Combating hyperthermia in acute stroke: A significant clinical concern, Stroke 29, 529–534 (1998).
H. Minamisawa, M. L. Smith, and B. K. Siesjo, The effect of mild hyperthermia and hypothermia on brain damage following 5, 10, and 15 minutes of forebrain ischemia, Ann. Neurol. 28, 26–33 (1990).
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2005 Springer Science+Business Media, Inc.
About this paper
Cite this paper
Nemoto, E.M., Jungreis, C., Larnard, D., Kuwabara, H., Horowitz, M., Kassam, A. (2005). Hyperthermia and Hypermetabolism in Focal Cerebral Ischemia. In: Okunieff, P., Williams, J., Chen, Y. (eds) Oxygen Transport to Tissue XXVI. Advances in Experimental Medicine and Biology, vol 566. Springer, Boston, MA. https://doi.org/10.1007/0-387-26206-7_12
Download citation
DOI: https://doi.org/10.1007/0-387-26206-7_12
Publisher Name: Springer, Boston, MA
Print ISBN: 978-0-387-25062-5
Online ISBN: 978-0-387-26206-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)