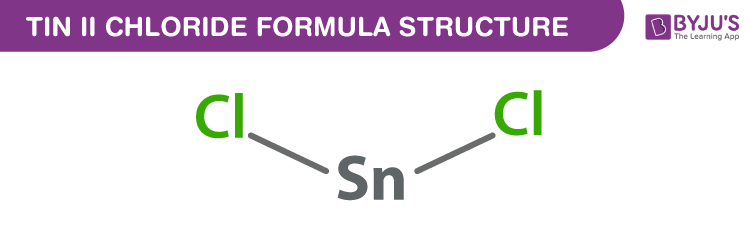
Tin (II) chloride formula, also named as Stannous chloride formula or Tin protochloride formula is discussed in this article. It consists of one atom of tin and two atoms of chlorine. The molecular or chemical formula of Tin (II) chloride is SnCl2.
Tin protochloride is a crystalline solid mass or flaky solid. It has a fatty appearance. It is capable of burning but faces difficulty to ignite. It is toxic when ingested and irritates eyes and skin. It is widely used in the manufacturing of dyes, pharmaceuticals products. It is also used as a tanning agent.
Tin (II) chloride Formula Structure
Properties Of Tin (II) chloride Formula
| Chemical formula | SnCl2 |
| Molecular weight | 189.60 g/mol (anhydrous)
225.63 g/mol (dihydrate) |
| Density | 3.95 g/cm3 (anhydrous) 2.71 g/cm3 (dihydrate) |
| Melting point | 247 °C (anhydrous)
37.7 °C (dihydrate) |
| Boiling point | 623 °C |
Anhydrous Tin (II) chloride is prepared by the treating dry hydrogen chloride gas on a tin metal. To make the dihydrate it is made to react with hydrochloric acid. The water is evaporated from the acidic solution to obtain crystals of SnCl2·2H2O. The dihydrate is dehydrated to anhydrous with the help of acetic anhydride.
To learn more about Tin(II) chloride formula from the expert faculties at BYJU’S, register now! Also, you can download notes on Stannous chloride for free.

Comments