Sodium sulfide is a chemical compound with the formula Na2S. They are colourless and when dissolved in water give a strongly alkaline solution. When exposed to moist air, sodium sulfide and its hydrates emit strong hydrogen sulfides that smells like rotten eggs. Industrially, sodium sulfide is produced by the carbothermic reaction of sodium sulfate using coal. In this short piece of article, let us learn more about the sodium sulfate formula and its chemical structure along with the various uses of sodium sulfide.
Sodium Sulfide Properties
| Properties of Sodium Sulfide | |
| Name | Sodium Sulfide |
| Appearance | Colourless Hygroscopic solid |
| Molecular Formula | Na2S |
| Melting Point | 1,176 °C |
| Density | 1.86 g/cm³ |
| Molar Mass | 78.0452 g/mol |
| Solubility in Water | Soluble |
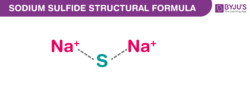
Sodium Sulfide Chemical Structure
Sodium Sulfide Uses
- Used in the kraft process in the paper and pulp industry
- Used as an oxygen scavenger agent in water treatment
- Used as a bleaching agent in the textile industry
- Used as an unhairing agent in the liming process during leather processing
Safety Measures
- Sodium sulfide is strong alkaline in nature it causes skin burns.
- Sodium Sulfides reacts with acid very rapidly to produce hydrogen sulfide which is toxic.
To learn more about such chemistry topics register to BYJU’S now!

Comments