Magnesium Nitride Formula formula, also known as Trimagnesium Dinitride formula or Trimagnesium Nitrogen (-3) Anion formula is explained in this article. It is an inorganic compound of magnesium and nitrogen consisting of 3 atoms of Magnesium and two atoms of nitrogen. The chemical or molecular formula of Magnesium Nitride Formula is Mg3N2.
It occurs as a greenish to yellow powder at room temperature and pressure. It dissolves in acid, slightly soluble in ether and ethanol. It can be prepared by passing dry nitrogen on heated magnesium. It can also be prepared by passing ammonia on heated magnesium. When Magnesium Nitride is Thermal decomposed thermally it generates nitrogen and magnesium gas.
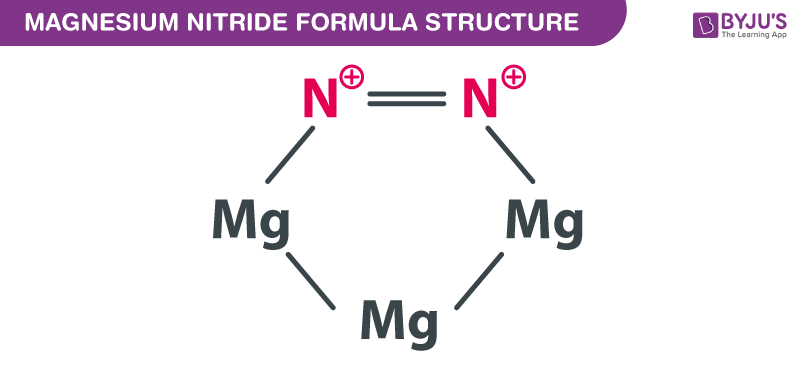
Magnesium Nitride Formula Structure
Properties Of Magnesium Nitride
| Chemical formula | Mg3N2 |
| Molecular weight | 100.9494 g/mol |
| Density | 2.712 g/cm3 |
| Appears as | Powder |
| Melting point | Approximately 1500 °C |
It can be used to prepare other nitride compounds with corrosion resistance, high hardness, wear resistance, high-temperature resistance, and high thermal conductivity properties. In the first practical synthesis of borazon, magnesium nitride was used as the catalyst.
To learn more about Magnesium Nitride Formula from the expert faculties at BYJU’S, register now!

Comments