Dinitrogen monoxide, also known as nitrous oxide, is an inorganic compound with a chemical formula N2O. It is colourless and inflammable at room temperature with a slight taste and scent of metals. It acts as a powerful oxidizer quite similar to the molecular oxygen at elevated temperature. In this short piece of article, let us discuss the dinitrogen monoxide formula, its chemical structure, properties and uses of dinitrogen monoxide.
Nitrous Oxide Properties
| Properties of Dinitrogen Monoxide | |
| Name | Dinitrogen Monoxide |
| Also Known as | Nitrous Oxide, Dinitrogen oxide, and Laughing gas |
| Appearance | Colourless Gas |
| Molecular Formula | N2O |
| Melting Point | −88.48 °C |
| Boiling Point | −90.86 °C |
| Density | 1.98 kg/m³ |
| Molar Mass | 44.013 g/mol |
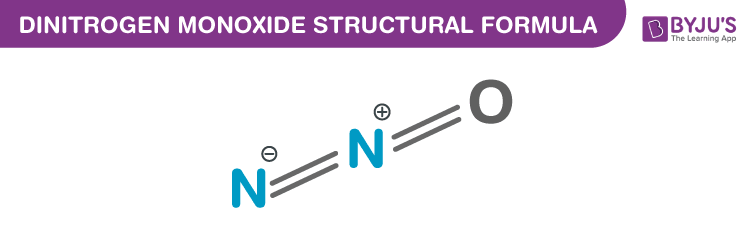
Dinitrogen Monoxide chemical structure
Nitrous Oxide Uses
- Used as a medical anesthetic and analgesic
- Used as a propellant for dispensing fatty liquids
- Used as a fuel additive in ca racing
To learn more about such chemistry topics register to BYJU’S now!

Comments