The chloroacetic acid formula, also named as Monochloroacetic acid formula or Chloroethanoic acid formula is discussed in this article. It is an organochlorine compound and is considered as an effective building-block. The molecular or chemical formula of Chloroacetic acid is C2H3ClO2. The other names of chloroacetic acid are 2-chloroacetic acid, 2-chloroethanoic acid.
In its solid form, it appears as colourless to light-brown crystals. It sinks as well as dissolves in water. In its aqueous form, it appears as a colourless solution. The concentration of acid can be around 80%. It is widely used as a herbicide, bacteriostat, and preservative.
Monochloroacetic acid is synthesized industrially through two processes.
- The first process carries the chlorination of acetic acid in the presence of catalyst acetic anhydride.
- The second process involves hydrolysis of trichloroethylene in the presence of a catalyst sulfuric acid (H2SO4).
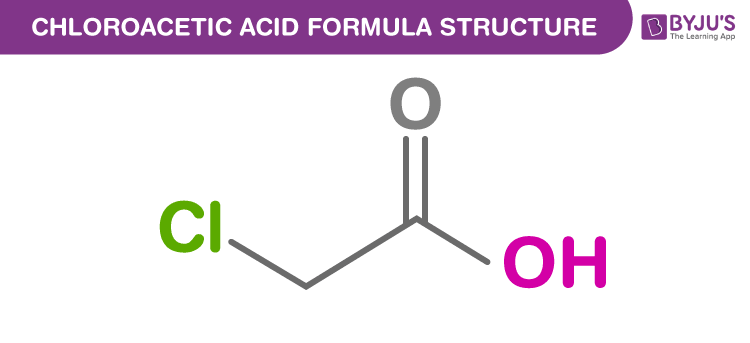
Chloroacetic acid Formula Structure
Properties Of Chloroacetic acid Formula
| Chemical formula | C2H3ClO2 |
| Molecular weight | 94.49 g/mol |
| Density | 1.58 g/cm3 |
| Melting point | 63 °C |
| Boiling point | 189.3 °C |
Safety Measures
- It is combustible.
- It is toxic and corrosive to tissues and metals.
- It is harmful if by ingested, inhaled or absorbed through the skin.
- It can cause thermal burns.
To learn more about Chloroacetic acid formula from the expert faculties at BYJU’S, register now! Also, you can download notes on Monochloroacetic acid for free.

Comments