What is Sodium Bromide?
Sodium bromide is an inorganic compound in its dry form a white crystalline powder with a salty and somewhat bitter taste. The chemical formula for sodium bromide in NaBr. It is a white crystal or white, granular powder having the odour of sulphur dioxide. It does not occur as a natural solid due to its solubility, it is extracted from ocean water along with chlorides, iodides and halites. It possesses anticonvulsant properties of any bromide salt and is one of the most common salts of hydrobromic acid.
Other names – Bromide salt of sodium
| NaBr | Sodium Bromide |
| Density | 3.21 g/cm³ |
| Molecular weight/ Molar mass | 102.894 g/mol |
| Boiling point | 1,396 °C |
| Melting point | 747 °C |
| Chemical formula | NaBr |
Table of Contents
- Sodium Bromide Structure – NaBr
- Physical Properties of Sodium Bromide – NaBr
- Chemical Properties of Sodium Bromide – NaBr
- Uses of Sodium Bromide – NaBr
- Health Hazard
- Frequently Asked Questions – FAQs
Sodium Bromide Structure – NaBr
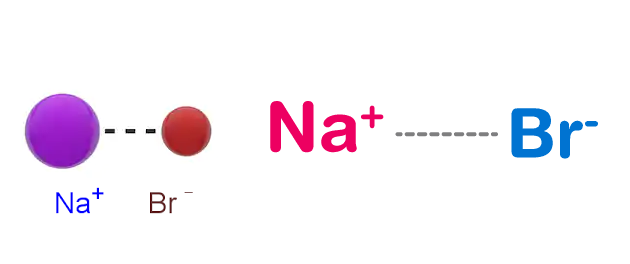
Physical Properties of Sodium Bromide – NaBr
| Odour | No odour |
| Appearance | White, crystalline solid |
| Heat capacity | 298.15 K, J/mol·K |
| Complexity | 2 |
| Solubility | Readily soluble in water |
Chemical Properties of Sodium Bromide – NaBr
-
- Sodium bromide reacts with sulphuric acid to form sodium sulphate and hydrogen bromide. The chemical equation is given below.
NaBr + H2SO4 → NaHSO4 + HBr
-
- Sodium bromide reacts with silver nitrate solution forms silver bromide and sodium nitrate. The chemical equation is given below.
Uses of Sodium Bromide – NaBr
- Used as a sedative-like other bromides.
- Used in the oil and gas drilling industry is a principal consumer of sodium bromide.
- Used for its germicidal properties due to liberation of bromine.
- Used as an antiseptic, detergent, and as reagent in pharmaceutical preparations.
Health Hazard
Skin rashes are common, and high blood levels of bromides may cause serious neurologic and psychologic disturbances. The great danger of toxicity in patients on low salt diets. Sodium bromide is moderately toxic by ingestion and can affect the gastrointestinal and central nervous systems.
Frequently Asked Questions
Is sodium bromide solid, liquid, or gaseous?
Sodium bromide is a white-coloured solid. This quickly dissolves into the mud. It produces bromine gas when heated very strongly in air. This also reacts to generate liquid bromine with chlorine.
Is sodium bromide covalent or ionic?
Sodium bromide is an ionically bonded compound. The electronegativity of bromine is high enough and the electromagnetic force between the Br and the Na atoms is great enough that an electron is transferred from the Na atom to the Br atom. Therefore, bromine becomes negatively charged and sodium becomes positively charged.
What is sodium bromide used for?
Sodium bromide, also known as Sedoneural, can be used as a hypnotic, as an anticonvulsant, and as a sedative. In the field of medicine, it is widely used as anticonvulsant and a sedative in the late 19th and early 20th centuries.

Comments