What is Potassium sulfate?
K2SO4 is an inorganic chemical compound. It is also known as Sulfuric acid dipotassium salt or dipotassium sulfate. Potassium sulfate occurs naturally in volcanic lava and salt lakes. It appears as a colourless to white crystalline powder or crystals. It is odourless and has a bitter, hard and saline-like taste. It dissolves in water but insoluble in ethanol.
Table of contents
- Structure Of Potassium Sulfate
- Properties Of Potassium Sulfate – K2so4
- Production Of Potassium Sulfate
- Uses Of Potassium Sulfate
- FAQs
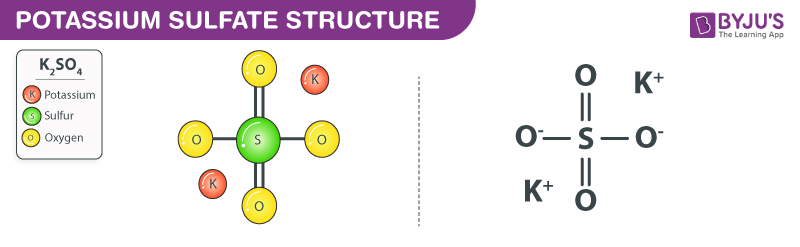
Potassium sulfate structure – K2SO4
Properties of Potassium sulfate – K2SO4
| K2SO4 | Potassium sulfate |
| Molecular Weight/ Molar Mass | 174.259 g/mol |
| Density | 2.66 g/cm³ |
| Boiling Point | 1,689 °C |
| Melting Point | 1,069 °C |
Production of potassium sulfate -K2SO4
The steps followed to obtain this compound are as follows:
- Crushing the mineral langbeinite
- Washing it
- Extracting the mineral
- Separating
The product is then treated with an aqueous solution of potassium chloride to separate the 2 parts of the double salt from each other.
Potassium sulfate compound can also be produced synthetically. This is possible by treating potassium chloride with raw sulfuric acid.
K2SO4 Uses (Potassium sulfate)
- It is dominantly used as a fertilizer for crops which include tobacco, some vegetables, and fruits.
- Potassium sulfate is used as a salt substitute.
- It is used in artillery propellant charges as a flash reducer.
- It is used in soda blasting.
- Potassium sulfate is used as a supplement for animal feeds.
- It is used in the production of lubricants and dyes.
- It is used in the manufacturing of ceramics and glass.
- It is used in the production of gypsum boards.
- It is used to synthesize potassium aluminium sulfate.
- It is used to produce gypsum cement.
- It is used in explosives as a flash suppressant
Frequently Asked Questions – FAQs
What is potassium sulphate used for?
Potassium sulfate is used as artillery propellant charges as a flash reducer. It is used in the manufacturing of ceramics , glass, production of gypsum boards and explosives as a flash suppressant
Why is potassium sulphate used in fertilizers?
Potassium sulfate is a source of potassium for plants. It is a common potash fertilizer. Potassium (K) is an essential element for plant growth; it is important to food crops. Potassium, often called potash, helps plants use water and resists drought and enhances fruits and vegetables.
What is the best source of potassium for plants?
Compost made primarily from food byproducts is an excellent source of potassium. In particular, banana peels are very high in potassium. Wood ash can also be used for potassium, but excess wood ash can burn the plants.
What are the symptoms of potassium deficiency?
A small drop in potassium level often does not cause symptoms, which may be mild, and may include:Constipation, Feeling of skipped heartbeats or palpitations, Fatigue, Muscle damage etc.
Is Potassium Sulphate good for plants?
Potassium sulfate is considered a premium-quality potash. It contains two key nutrients for growing crops: potassium and sulfur. Using potassium sulfate not only improves quality and crop yields, but also makes plants more resilient to drought, frost, insects and even disease.
Other important links:
| Potassium | Uses of Methanol and Ethanol |

Comments