All the important differences between soaps and detergents are explained in this article. Soaps are the potassium or sodium salts of long-chain fatty acids and detergents are generally alkyl benzene sulfonates.
What are Soaps and Detergents?
Soaps
- Soaps are potassium or sodium salts of a carboxylic acid having a long aliphatic chain attached to it.
- They are surfactants (compounds that reduce the surface tension between a liquid and another substance) and therefore help in the emulsification of oils in water.
- Soaps are generally prepared via the saponification of fats and oils.
- The carboxylate end of the soap molecule is hydrophilic whereas the hydrocarbon tail is hydrophobic.
Detergents
- Detergents are the potassium or sodium salts of a long alkyl chain ending with a sulfonate group.
- They are soluble in hard water.
- This solubility is attributed to the fact that the sulfonate group does not attach itself to the ions present in hard water.
- Commonly, anionic detergents such as alkyl benzene sulfonates are used for domestic purposes.
Recommended Videos on Soaps and Detergents
Difference between Soap and Detergent
The key differences between soaps and detergents are tabulated below.
| Difference Between Soap and Detergent | |
| Soaps | Detergents |
| Consist of a ‘-COONa’ group attached to a fatty acid having a long alkyl chain. | Consist of a ‘-SO3Na’ group attached to a long alkyl chain. |
| They are not effective in hard water and saline water | They do not lose their effectiveness in hard water and saline water. |
| Soaps are completely biodegradable | Detergents containing a branched hydrocarbon chain are non-biodegradable |
| They have a tendency to form scum in a hard water environment. | These compounds do not form scum. |
| They are derived from natural sources such as vegetable oils and animal fats. | Detergents are synthetic derivatives. |
| Soaps are environment-friendly products since they are biodegradable. | These compounds can form a thick foam that causes the death of aquatic life. |
| Examples of soaps: sodium palmitate and sodium stearate. | Examples of detergents: deoxycholic acid and sodium lauryl sulfate. |
Preparation of Soap
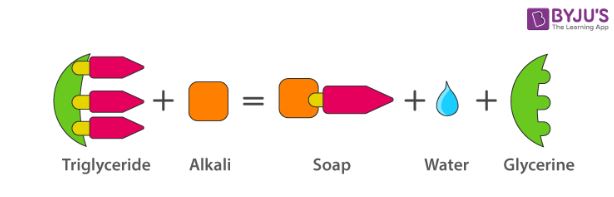
The most commonly used soap making process is the saponification of oils and fats.
This process involves heating oils and fats reacting them with a liquid alkali to produce soap plus water plus glycerine.
Saponification
The other soap making process is the neutralization of fatty acids with an alkali. Oils and fats are hydrolyzed with high-pressure steam to yield glycerine and crude fatty acids.
Saponification
The fatty acids are later purified by the method of distillation and neutralized with an alkali to produce water and soap.
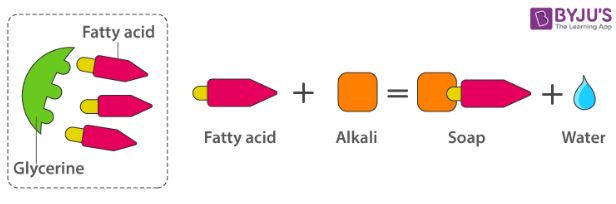
Preparation of Soap
Alkali like sodium hydroxide produces sodium soap which is hard. Potassium soaps are soft. They are used in shaving creams and some liquid hand soaps.
The carboxylate end of the soap molecule is a hydrophilic end. The grease and oil attract the hydrocarbon chain and repel water. This is known as the hydrophobic end.
How do Soaps and Detergents Clean out Dirt?
Cleaning a soiled surface is a four-step process. In the first step, the surface to be cleaned is made wet with water. In the second step, soap or detergent is applied to the surface to be absorbed.
Soaps and detergents are also called surface-active agents, or surfactants. Surface active molecules present in soaps and detergents dissolve in water. This solution serves to loosen surface tension or the force that holds together molecules on a surface or on cloth. When this happens, it helps water to spread easily over a surface or soak into clothes.
In the third step, when clothes are rubbed together, either by hand or in a washing machine, dirt particles are broken up as surface-active molecules work to separate the dirt from clothes and deposit them in the water. In the fourth and final step of the cleaning process, the separated dirt is prevented from going and re-depositing on the surface of clean clothes. Dirt particles are coated with soap and detergent molecules. This keeps them suspended in water until the dirt is washed away with rinsing.
Frequently Asked Questions – FAQs
What is the chemical process involved in soap?
Soaps are surfactants which means they dissolve and can clean in water and oils. Soapmaking involves reacting fats/oils with a solid base of hydroxide, to form glycerin and soap (fatty acid salts). The fat/oil molecules (triglycerides) consist of glycerin that is chemically bound to three fatty acids.
Are soaps and detergents basic?
Soaps are fatty acid salts which are water-soluble sodium or potassium. Soaps are made from fats and oils, or their fatty acids, by chemically treating them with a heavy alkali. So, Salts are soaps and detergents. Because of this, they have very simple properties in them and can be regarded as simple salts.
What is the difference between soap and detergent?
Soaps are the sodium salts of carboxylic acids in long chains. Sodium salts of long-chain benzene sulphonic acids are detergents. Soaps are biodegradable while some of the detergents can not be biodegraded. Soaps have relatively weak cleaning action, whereas detergents have a strong cleaning effect.
What is the chemical reaction of soap?
Saponification is the term for the soap-producing chemical reaction. Animal or vegetable fat is converted to soap (a fatty acid) and alcohol during the process. The reaction requires an alkali solution in water and also heat (e.g., sodium hydroxide or potassium hydroxide).
What are the properties of soap?
Soaps are water-soluble, fatty acid sodium salts. Soaps are made of fats and oils, or they are fatty acids, using solid alkali (a base) to handle them. The most widely used process for making soap is the making of fats and oils.
To learn more about soaps and detergents, register with BYJU’S and download the mobile application on your smartphone.

thank u, i got what i need.
Informative Writing
What makes soap biodegradable?
Soaps are usually deemed biodegradable if bacteria can break them down to at least 90-percent water, CO2, and organic material within six months. This simple step ensures you aren’t adding anything unnecessary to the land and waterways while you are out there enjoying them.