What is Methylation?
Methylation refers to the addition of a methyl group (CH3 group) to a compound or the substitution of one of its functional groups with a methyl group. It can be considered as a specific type of alkylation in which only a CH3 group is transferred. An illustration detailing the methylation of cytosine (one of the four major bases found in DNA and RNA) is provided below.
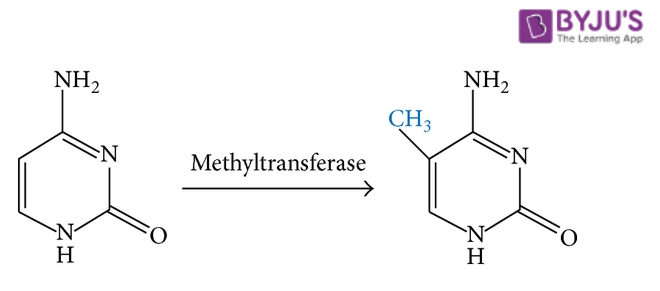
It can be noted that the opposite process (i.e. the removal of a methyl group from a compound) is known as demethylation.
Table of Contents
- Electrophilic Methylation
- Nucleophilic Methylation
- The Eschweiler-Clarke Reaction
- Frequently Asked Questions-FAQs
Electrophilic Methylation
In electrophilic methylations, the source of the methyl group is an electrophile. Examples of electrophilic methylating agents include iodomethane, dimethyl carbonate, dimethyl sulphate, and diazomethane. In these reactions, the methylation proceeds via an SN2 reaction.
Examples of chemical reactions that can be classified as electrophilic methylation reactions include:
- The methylation of a carboxylate ion at its oxygen to afford a methyl ester.
- The formation of an ether from the methylation of an alkoxide ion.
- The methylation of the enolate of a ketone to yield a new ketone.
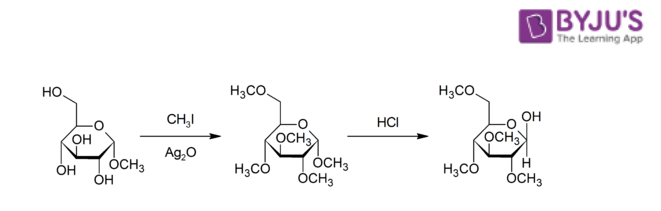
- The Purdie methylation reaction in which the oxygen belonging to some carbohydrate is methylated with the help of silver oxide and iodomethane.
Nucleophilic Methylation
When a nucleophilic methylating agent is used for the transfer of a CH3 group, the process is referred to as nucleophilic methylation. Examples of nucleophilic methylating agents include:
- Grignard reagents in which the R group is a methyl group (such as CH3-Mg-Br)
- Methyllithium (CH3Li)
- Tetramethyltin ((CH3)4Sn)
- Trimethyl aluminium (Al2(CH3)6)
- Dimethyl zinc (Zn(CH3)2)
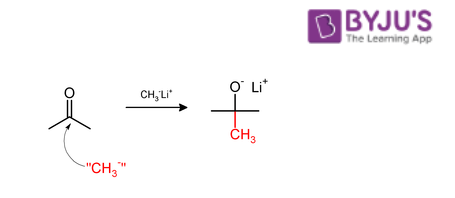
When aldehydes and ketones are subjected to methylation with the help of a nucleophilic methylating agent, the CH3 group is usually added to the carbonyl carbon (as illustrated below).
The Eschweiler-Clarke Reaction
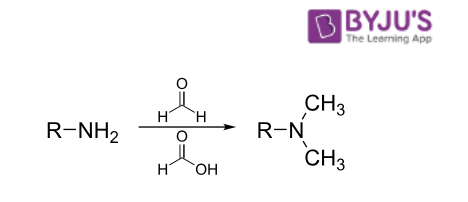
In this named reaction, a primary/secondary amine undergoes methylation upon its treatment with formaldehyde and an excess of formic acid. The general format of this reaction is illustrated below.
To learn more about methylation and other important categories of organic reactions (such as acetylation), register with BYJU’S and download the mobile application on your smartphone.
The mechanism of methylation allows one to perform both physically and psychologically, so it should not be shocking that this method is used for several different functions of the body. Such functions include the functioning of the nervous, cardiovascular and immune systems,5-8 as well as the processing of oxygen, detoxification of heavy metals and hormone regulation.
Frequently Asked Questions – FAQs
What is meant by the term ‘methylation’?
The term ‘methylation’ is used to refer to the addition of a methyl group to a given reactant. It can also refer to the substitution of one of the functional groups on a given reactant with a methyl group. The process of methylation can be thought of as a type of alkylation in which a methyl group is interchanged with a hydrogen atom on a substrate.
What is the role of methylation in biological systems?
Methylation reactions are frequently used in the regulation of gene expression and the regulation of protein function in biological systems. It can be noted that the methylations that occur in the bodies of living things are catalysed by enzymes. It can also be noted that methylation also plays a role in RNA processing in biological systems.
What is methanogenesis?
Methanogenesis, also known as biomethanation, is a process through which methane is generated from carbon dioxide via multiple methylation reactions. Such reactions usually occur in certain anaerobic microbes that contain a specific set of enzymes. It can be noted that the reverse of this reaction, known as reverse methanogenesis, features the use of methane as the methylating agent.
What is electrophilic methylation?
Electrophilic methylation is a class of methylation reactions in which the methyl group sources are electrophilic in nature. Common examples of electrophilic methyl sources include dimethyl sulfate, iodomethane, and dimethyl carbonate. It is important to note that these reagents participate in methylations via SN2 reactions (a class of nucleophilic substitution reactions).
What is the Eschweiler-Clarke methylation reaction?
The Eschweiler-Clarke methylation reaction is a named reaction in which amines are subjected to methylation. The primary advantage of this method is that it avoids the chance for quaternization. This reaction is named after the British chemist Hans Thacher Clarke and the German chemist Wilhelm Eschweiler.
