Interaction between cyclin-dependent kinases and human papillomavirus replication-initiation protein E1 is required for efficient viral replication
- PMID: 9892642
- PMCID: PMC15145
- DOI: 10.1073/pnas.96.2.382
Interaction between cyclin-dependent kinases and human papillomavirus replication-initiation protein E1 is required for efficient viral replication
Abstract
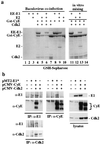
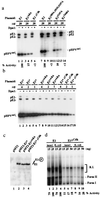
We have identified the human papillomavirus (HPV) DNA replication initiation protein E1 as a tight-binding substrate of cyclin E/cyclin-dependent kinase (Cdk) complexes by using expression cloning. E1, a DNA helicase, collaborates with the HPV E2 protein in ori-dependent replication. E1 formed complexes with cyclin E in insect and mammalian cells, independent of Cdks and E2. Additional cyclins, including A-, B-, and F-type (but not D-type), interacted with the E1/E2 complex, and A- and E-type cyclin kinases were capable of phosphorylating E1 and E2 in vitro. Association with cyclins and efficient phosphorylation of E1 required the presence of a cyclin interaction motif (the RXL motif). E1 lacking the RXL motif displayed defects in E2-dependent HPV ori replication in vivo. Consistent with a role for Cdk-mediated phosphorylation in E1 function, an E1 protein lacking all four candidate Cdk phosphorylation sites still associated with E2 and cyclin E but was impaired in HPV replication in vitro and in vivo. Our data reveal a link between cyclin/Cdk function and activation of HPV DNA replication through targeting of Cdk complexes to the E1 replication-initiation protein and suggest a functional role for E1 phosphorylation by Cdks. The use of cyclin-binding RXL motifs is now emerging as a major mechanism by which cyclins are targeted to key substrates.
Figures
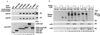

Similar articles
-
HeLa cells are phenotypically limiting in cyclin E/CDK2 for efficient human papillomavirus DNA replication.J Biol Chem. 2000 Mar 3;275(9):6167-74. doi: 10.1074/jbc.275.9.6167. J Biol Chem. 2000. PMID: 10692408
-
Cyclin/CDK regulates the nucleocytoplasmic localization of the human papillomavirus E1 DNA helicase.J Virol. 2004 Dec;78(24):13954-65. doi: 10.1128/JVI.78.24.13954-13965.2004. J Virol. 2004. PMID: 15564503 Free PMC article.
-
Nucleocytoplasmic shuttling of bovine papillomavirus E1 helicase downregulates viral DNA replication in S phase.J Virol. 2007 Jan;81(1):384-94. doi: 10.1128/JVI.01170-06. Epub 2006 Oct 11. J Virol. 2007. PMID: 17035309 Free PMC article.
-
Papillomavirus E1 proteins: form, function, and features.Virus Genes. 2002 Jun;24(3):275-90. doi: 10.1023/a:1015336817836. Virus Genes. 2002. PMID: 12086149 Review.
-
Cyclin E2, the cycle continues.Int J Biochem Cell Biol. 2002 Apr;34(4):315-20. doi: 10.1016/s1357-2725(01)00137-6. Int J Biochem Cell Biol. 2002. PMID: 11854029 Review.
Cited by
-
Genomic Characterisation of Canis Familiaris Papillomavirus Type 24, a Novel Papillomavirus Associated with Extensive Pigmented Plaque Formation in a Pug Dog.Viruses. 2022 Oct 26;14(11):2357. doi: 10.3390/v14112357. Viruses. 2022. PMID: 36366455 Free PMC article.
-
Analysis of coevolution in nonstructural proteins of chikungunya virus.Virol J. 2016 Jun 2;13:86. doi: 10.1186/s12985-016-0543-1. Virol J. 2016. PMID: 27251040 Free PMC article.
-
The differential requirement for cyclin-dependent kinase activities distinguishes two functions of herpes simplex virus type 1 ICP0.J Virol. 2003 Dec;77(23):12603-16. doi: 10.1128/jvi.77.23.12603-12616.2003. J Virol. 2003. PMID: 14610183 Free PMC article.
-
Activity of CK2α protein kinase is required for efficient replication of some HPV types.PLoS Pathog. 2019 May 15;15(5):e1007788. doi: 10.1371/journal.ppat.1007788. eCollection 2019 May. PLoS Pathog. 2019. PMID: 31091289 Free PMC article.
-
Manipulation of cellular DNA damage repair machinery facilitates propagation of human papillomaviruses.Semin Cancer Biol. 2014 Jun;26:30-42. doi: 10.1016/j.semcancer.2013.12.003. Epub 2014 Jan 8. Semin Cancer Biol. 2014. PMID: 24412279 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

