A steep dependence of inward-rectifying potassium channels on cytosolic free calcium concentration increase evoked by hyperpolarization in guard cells
- PMID: 9880370
- PMCID: PMC32230
- DOI: 10.1104/pp.119.1.277
A steep dependence of inward-rectifying potassium channels on cytosolic free calcium concentration increase evoked by hyperpolarization in guard cells
Abstract
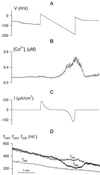
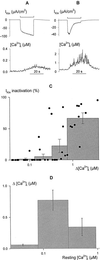
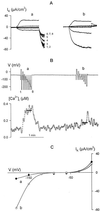
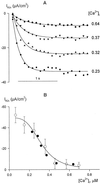
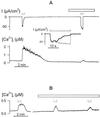
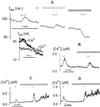
Inactivation of inward-rectifying K+ channels (IK,in) by a rise in cytosolic free [Ca2+] ([Ca2+]i) is a key event leading to solute loss from guard cells and stomatal closure. However, [Ca2+]i action on IK,in has never been quantified, nor are its origins well understood. We used membrane voltage to manipulate [Ca2+]i (A. Grabov and M.R. Blatt [1998] Proc Natl Acad Sci USA 95: 4778-4783) while recording IK,in under a voltage clamp and [Ca2+]i by Fura-2 fluorescence ratiophotometry. IK,in inactivation correlated positively with [Ca2+]i and indicated a Ki of 329 +/- 31 nM with cooperative binding of four Ca2+ ions per channel. IK,in was promoted by the Ca2+ channel antagonists Gd3+ and calcicludine, both of which suppressed the [Ca2+]i rise, but the [Ca2+]i rise was unaffected by the K+ channel blocker Cs+. We also found that ryanodine, an antagonist of intracellular Ca2+ channels that mediate Ca2+-induced Ca2+ release, blocked the [Ca2+]i rise, and Mn2+ quenching of Fura-2 fluorescence showed that membrane hyperpolarization triggered divalent release from intracellular stores. These and additional results point to a high signal gain in [Ca2+]i control of IK,in and to roles for discrete Ca2+ flux pathways in feedback control of the K+ channels by membrane voltage.
Figures
Similar articles
-
Extracellular nucleotides activate non-selective cation and Ca(2+)-dependent K+ channels in rat osteoclasts.J Physiol. 1997 Sep 1;503 ( Pt 2)(Pt 2):303-15. doi: 10.1111/j.1469-7793.1997.303bh.x. J Physiol. 1997. PMID: 9306274 Free PMC article.
-
Kinetic and pharmacological properties of the M-current in rodent neuroblastoma x glioma hybrid cells.J Physiol. 1992;451:159-85. doi: 10.1113/jphysiol.1992.sp019159. J Physiol. 1992. PMID: 1403809 Free PMC article.
-
Sensitivity to abscisic acid of guard-cell K+ channels is suppressed by abi1-1, a mutant Arabidopsis gene encoding a putative protein phosphatase.Proc Natl Acad Sci U S A. 1995 Oct 10;92(21):9520-4. doi: 10.1073/pnas.92.21.9520. Proc Natl Acad Sci U S A. 1995. PMID: 7568166 Free PMC article.
-
K+ channels of stomatal guard cells. Characteristics of the inward rectifier and its control by pH.J Gen Physiol. 1992 Apr;99(4):615-44. doi: 10.1085/jgp.99.4.615. J Gen Physiol. 1992. PMID: 1534573 Free PMC article.
-
Signal transduction and ion channels in guard cells.Philos Trans R Soc Lond B Biol Sci. 1998 Sep 29;353(1374):1475-88. doi: 10.1098/rstb.1998.0303. Philos Trans R Soc Lond B Biol Sci. 1998. PMID: 9800209 Free PMC article. Review.
Cited by
-
Systems dynamic modeling of the stomatal guard cell predicts emergent behaviors in transport, signaling, and volume control.Plant Physiol. 2012 Jul;159(3):1235-51. doi: 10.1104/pp.112.197350. Epub 2012 May 25. Plant Physiol. 2012. PMID: 22635112 Free PMC article.
-
Paxillus involutus-Facilitated Cd2+ Influx through Plasma Membrane Ca2+-Permeable Channels Is Stimulated by H2O2 and H+-ATPase in Ectomycorrhizal Populus × canescens under Cadmium Stress.Front Plant Sci. 2017 Jan 6;7:1975. doi: 10.3389/fpls.2016.01975. eCollection 2016. Front Plant Sci. 2017. PMID: 28111579 Free PMC article.
-
Dual function of overexpressing plasma membrane H+-ATPase in balancing carbon-water use.Sci Adv. 2024 Nov 8;10(45):eadp8017. doi: 10.1126/sciadv.adp8017. Epub 2024 Nov 8. Sci Adv. 2024. PMID: 39514663 Free PMC article.
-
Induction of abscisic acid-regulated gene expression by diacylglycerol pyrophosphate involves Ca2+ and anion currents in Arabidopsis suspension cells.Plant Physiol. 2006 Aug;141(4):1555-62. doi: 10.1104/pp.106.080218. Epub 2006 Jun 9. Plant Physiol. 2006. PMID: 16766676 Free PMC article.
-
Extracellular protons inhibit the activity of inward-rectifying potassium channels in the motor cells of Samanea saman pulvini.Plant Physiol. 2001 Nov;127(3):1310-22. Plant Physiol. 2001. PMID: 11706209 Free PMC article.
References
-
- Allen GJ, Muir SR, Sanders D. Release of Ca2+ from individual plant vacuoles by both insp(3) and cyclic ADP-ribose. Science. 1995;268:735–737. - PubMed
-
- Allen GJ, Sanders D. Control of ionic currents in guard cell vacuoles by cytosolic and luminal calcium. Plant J. 1996;10:1055–1069. - PubMed
-
- Armstrong F, Blatt MR. Evidence for K+ channel control in Vicia guard cells coupled by G-proteins to a 7TMS receptor. Plant J. 1995;8:187–198.
LinkOut - more resources
Full Text Sources
Miscellaneous

