Antigen-dependent CD28 signaling selectively enhances survival and proliferation in genetically modified activated human primary T lymphocytes
- PMID: 9705944
- PMCID: PMC2213361
- DOI: 10.1084/jem.188.4.619
Antigen-dependent CD28 signaling selectively enhances survival and proliferation in genetically modified activated human primary T lymphocytes
Abstract
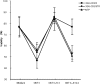
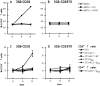
Most tumor cells function poorly as antigen-presenting cells in part because they do not express costimulatory molecules. To provide costimulation to T lymphocytes that recognize tumor cells, we constructed a CD28-like receptor specific for GD2, a ganglioside overexpressed on the surface of neuroblastoma, small-cell lung carcinoma, melanoma, and other human tumors. Recognition of GD2 was provided by a single-chain antibody derived from the GD2-specific monoclonal antibody 3G6. We demonstrate that the chimeric receptor 3G6-CD28 provides CD28 signaling upon specific recognition of the GD2 antigen on tumor cells. Human primary T lymphocytes retrovirally transduced with 3G6-CD28 secrete interleukin 2, survive proapoptotic culture conditions, and selectively undergo clonal expansion in the presence of an antiidiotypic antibody specific for 3G6-CD28. Polyclonal CD8(+) lymphocytes expressing 3G6-CD28 are selectively expanded when cultured with cells expressing allogeneic major histocompatibility complex class I together with GD2. Primary T cells given such an antigen-dependent survival advantage should be very useful to augment immune responses against tumor cells.
Figures
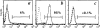
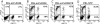
Similar articles
-
Different cytokine and stimulation conditions influence the expansion and immune phenotype of third-generation chimeric antigen receptor T cells specific for tumor antigen GD2.Cytotherapy. 2015 Apr;17(4):487-95. doi: 10.1016/j.jcyt.2014.12.002. Epub 2015 Jan 6. Cytotherapy. 2015. PMID: 25573334
-
4-1BB is superior to CD28 costimulation for generating CD8+ cytotoxic lymphocytes for adoptive immunotherapy.J Immunol. 2007 Oct 1;179(7):4910-8. doi: 10.4049/jimmunol.179.7.4910. J Immunol. 2007. PMID: 17878391 Free PMC article.
-
Costimulation of T cell activation by integrin-associated protein (CD47) is an adhesion-dependent, CD28-independent signaling pathway.J Exp Med. 1997 Jan 6;185(1):1-11. doi: 10.1084/jem.185.1.1. J Exp Med. 1997. PMID: 8996237 Free PMC article.
-
Redirected primary T cells harboring a chimeric receptor require costimulation for their antigen-specific activation.Blood. 2005 Apr 15;105(8):3087-93. doi: 10.1182/blood-2004-09-3737. Epub 2004 Dec 30. Blood. 2005. PMID: 15626734
-
Tumor-specific T cell activation by recombinant immunoreceptors: CD3 zeta signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3 zeta signaling receptor molecule.J Immunol. 2001 Dec 1;167(11):6123-31. doi: 10.4049/jimmunol.167.11.6123. J Immunol. 2001. PMID: 11714771
Cited by
-
Application of novel CAR technologies to improve treatment of autoimmune disease.Front Immunol. 2024 Oct 9;15:1465191. doi: 10.3389/fimmu.2024.1465191. eCollection 2024. Front Immunol. 2024. PMID: 39445021 Free PMC article. Review.
-
Current updates on generations, approvals, and clinical trials of CAR T-cell therapy.Hum Vaccin Immunother. 2022 Nov 30;18(6):2114254. doi: 10.1080/21645515.2022.2114254. Epub 2022 Sep 12. Hum Vaccin Immunother. 2022. PMID: 36094837 Free PMC article.
-
Anti-KIT designer T cells for the treatment of gastrointestinal stromal tumor.J Transl Med. 2013 Feb 21;11:46. doi: 10.1186/1479-5876-11-46. J Transl Med. 2013. PMID: 23433424 Free PMC article.
-
Cancer patient T cells genetically targeted to prostate-specific membrane antigen specifically lyse prostate cancer cells and release cytokines in response to prostate-specific membrane antigen.Neoplasia. 1999 Jun;1(2):123-7. doi: 10.1038/sj.neo.7900018. Neoplasia. 1999. PMID: 10933046 Free PMC article.
-
Treatment of lymphoma with adoptively transferred T cells.Expert Opin Biol Ther. 2009 Nov;9(11):1407-25. doi: 10.1517/14712590903260785. Expert Opin Biol Ther. 2009. PMID: 19723016 Free PMC article. Review.
References
-
- Nanda NK, Sercarz EE. Induction of anti-self-immunity to cure cancer. Cell. 1995;82:13–17. - PubMed
-
- Boon T, Coulie PD, Van den Eynde B. Tumor antigens recognized by T cells. Immunol Today. 1997;18:267–268. - PubMed
-
- Pardoll DM. New strategies for enhancing the immunogenicity of tumors. Curr Opin Immunol. 1993;5:719–725. - PubMed
-
- Baskar S. Gene-modified tumor cells as cellular vaccine. Cancer Immunol Immunother. 1996;43:165–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

