Activation of both MAP kinase and phosphatidylinositide 3-kinase by Ras is required for hepatocyte growth factor/scatter factor-induced adherens junction disassembly
- PMID: 9693375
- PMCID: PMC25472
- DOI: 10.1091/mbc.9.8.2185
Activation of both MAP kinase and phosphatidylinositide 3-kinase by Ras is required for hepatocyte growth factor/scatter factor-induced adherens junction disassembly
Abstract
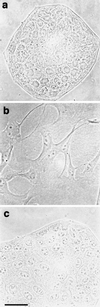
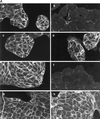
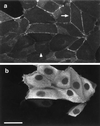
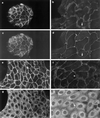
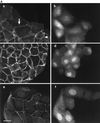
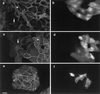
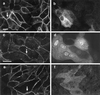
Hepatocyte growth factor/scatter factor (HGF/SF) stimulates the motility of epithelial cells, initially inducing centrifugal spreading of colonies followed by disruption of cell-cell junctions and subsequent cell scattering. In Madin-Darby canine kidney cells, HGF/SF-induced motility involves actin reorganization mediated by Ras, but whether Ras and downstream signals regulate the breakdown of intercellular adhesions has not been established. Both HGF/SF and V12Ras induced the loss of the adherens junction proteins E-cadherin and beta-catenin from intercellular junctions during cell spreading, and the HGF/SF response was blocked by dominant-negative N17Ras. Desmosomes and tight junctions were regulated separately from adherens junctions, because they were not disrupted by V12Ras. MAP kinase, phosphatidylinositide 3-kinase (PI 3-kinase), and Rac were required downstream of Ras, because loss of adherens junctions was blocked by the inhibitors PD098059 and LY294002 or by dominant-inhibitory mutants of MAP kinase kinase 1 or Rac1. All of these inhibitors also prevented HGF/SF-induced cell scattering. Interestingly, activated Raf or the activated p110alpha subunit of PI 3-kinase alone did not induce disruption of adherens junctions. These results indicate that activation of both MAP kinase and PI 3-kinase by Ras is required for adherens junction disassembly and that this is essential for the motile response to HGF/SF.
Figures




Similar articles
-
Phosphoinositide 3-kinase induces scattering and tubulogenesis in epithelial cells through a novel pathway.J Biol Chem. 1998 Jul 24;273(30):18793-801. doi: 10.1074/jbc.273.30.18793. J Biol Chem. 1998. PMID: 9668053
-
Inhibition of junction assembly in cultured epithelial cells by hepatocyte growth factor/scatter factor is concomitant with increased stability and altered phosphorylation of the soluble junctional molecules.Cell Growth Differ. 1997 Apr;8(4):451-62. Cell Growth Differ. 1997. PMID: 9101091
-
Phosphatidylinositol 3-kinase is required for adherens junction-dependent mammary epithelial cell spheroid formation.Differentiation. 2000 Oct;66(2-3):116-25. doi: 10.1046/j.1432-0436.2000.660206.x. Differentiation. 2000. PMID: 11100902
-
Cadherin-dependent organization and disorganization of epithelial architecture.Princess Takamatsu Symp. 1994;24:28-37. Princess Takamatsu Symp. 1994. PMID: 8983061 Review.
-
Molecular mechanisms leading to loss of differentiation and gain of invasiveness in epithelial cells.J Cell Sci Suppl. 1993;17:159-64. doi: 10.1242/jcs.1993.supplement_17.23. J Cell Sci Suppl. 1993. PMID: 8144693 Review.
Cited by
-
Activated protein C promotes protease-activated receptor-1 cytoprotective signaling through β-arrestin and dishevelled-2 scaffolds.Proc Natl Acad Sci U S A. 2011 Dec 13;108(50):E1372-80. doi: 10.1073/pnas.1112482108. Epub 2011 Nov 21. Proc Natl Acad Sci U S A. 2011. PMID: 22106258 Free PMC article.
-
The tyrosine phosphatase SHP-2 is required for sustained activation of extracellular signal-regulated kinase and epithelial morphogenesis downstream from the met receptor tyrosine kinase.Mol Cell Biol. 2000 Nov;20(22):8513-25. doi: 10.1128/MCB.20.22.8513-8525.2000. Mol Cell Biol. 2000. PMID: 11046147 Free PMC article.
-
Sequential activation of ERK and repression of JNK by scatter factor/hepatocyte growth factor in madin-darby canine kidney epithelial cells.Mol Biol Cell. 2000 Nov;11(11):3751-63. doi: 10.1091/mbc.11.11.3751. Mol Biol Cell. 2000. PMID: 11071904 Free PMC article.
-
Small GTPases and regulation of cadherin dependent cell-cell adhesion.Mol Pathol. 1999 Aug;52(4):197-202. doi: 10.1136/mp.52.4.197. Mol Pathol. 1999. PMID: 10694939 Free PMC article. Review.
-
Post-transcriptional down-regulation of ROCKI/Rho-kinase through an MEK-dependent pathway leads to cytoskeleton disruption in Ras-transformed fibroblasts.Mol Biol Cell. 2002 Jan;13(1):336-47. doi: 10.1091/mbc.01-06-0302. Mol Biol Cell. 2002. PMID: 11809843 Free PMC article.
References
-
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. - PubMed
-
- Avruch J, Zhang X-F, Kyriakis JM. Raf meets Ras: completing the framework of a signal transduction pathway. Trends Biochem Sci. 1994;19:279–283. - PubMed
-
- Balda MS, Whitney JA, Flores C, Gonzáles S, Cereijdo M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031–1049. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

