Human interferon-gamma-inducible protein 10 (IP-10) inhibits constitutive signaling of Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor
- PMID: 9670053
- PMCID: PMC2212452
- DOI: 10.1084/jem.188.2.405
Human interferon-gamma-inducible protein 10 (IP-10) inhibits constitutive signaling of Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor
Abstract
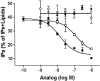
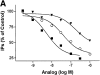
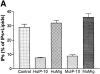
A G protein-coupled receptor (GPCR) is encoded within the genome of Kaposi's sarcoma- associated herpesvirus (KSHV)/human herpesvirus 8, a virus that may be involved in the pathogenesis of Kaposi's sarcoma and primary effusion lymphomas. KSHV-GPCR exhibits constitutive signaling activity that causes oncogenic transformation. We report that human interferon (IFN)-gamma-inducible protein 10 (HuIP-10), a C-X-C chemokine, specifically inhibits signaling of KSHV-GPCR. In contrast, monokine induced by IFN-gamma (HuMig), which like HuIP-10 is an agonist of C-X-C chemokine receptor 3, does not inhibit KSHV-GPCR signaling. Moreover, HuIP-10, but not HuMig, inhibits KSHV-GPCR-induced proliferation of NIH 3T3 cells. These results show that HuIP-10 is an inverse agonist that converts KSHV-GPCR from an active to an inactive state. Thus, a human chemokine inhibits constitutive signaling and cellular proliferation that is mediated by a receptor encoded by a human disease-associated herpesvirus.
Figures


Similar articles
-
Kaposi's sarcoma-associated herpesvirus (KSHV) chemokine vMIP-II and human SDF-1alpha inhibit signaling by KSHV G protein-coupled receptor.Biochem Biophys Res Commun. 1998 Dec 30;253(3):725-7. doi: 10.1006/bbrc.1998.9557. Biochem Biophys Res Commun. 1998. PMID: 9918794
-
Chemokines activate Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor in mammalian cells in culture.J Clin Invest. 1998 Oct 15;102(8):1469-72. doi: 10.1172/JCI4461. J Clin Invest. 1998. PMID: 9788958 Free PMC article.
-
Inhibition of constitutive signaling of Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor by protein kinases in mammalian cells in culture.J Exp Med. 1998 Mar 2;187(5):801-6. doi: 10.1084/jem.187.5.801. J Exp Med. 1998. PMID: 9480990 Free PMC article.
-
Cyclooxygenase-2-prostaglandin E2-eicosanoid receptor inflammatory axis: a key player in Kaposi's sarcoma-associated herpes virus associated malignancies.Transl Res. 2013 Aug;162(2):77-92. doi: 10.1016/j.trsl.2013.03.004. Epub 2013 Apr 6. Transl Res. 2013. PMID: 23567332 Free PMC article. Review.
-
Modulation of cell signaling pathways by Kaposi's sarcoma-associated herpesvirus (KSHVHHV-8).Cell Biochem Biophys. 2004;40(3):305-22. doi: 10.1385/CBB:40:3:305. Cell Biochem Biophys. 2004. PMID: 15211030 Review.
Cited by
-
Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi's sarcoma.J Exp Med. 2000 Feb 7;191(3):445-54. doi: 10.1084/jem.191.3.445. J Exp Med. 2000. PMID: 10662790 Free PMC article.
-
Inverse agonism at G protein-coupled receptors: (patho)physiological relevance and implications for drug discovery.Br J Pharmacol. 2000 May;130(1):1-12. doi: 10.1038/sj.bjp.0703311. Br J Pharmacol. 2000. PMID: 10780991 Free PMC article. Review. No abstract available.
-
Tissue Transglutaminase Promotes Early Differentiation of Oligodendrocyte Progenitor Cells.Front Cell Neurosci. 2019 Jul 2;13:281. doi: 10.3389/fncel.2019.00281. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31312122 Free PMC article.
-
Differential activation of murine herpesvirus 68- and Kaposi's sarcoma-associated herpesvirus-encoded ORF74 G protein-coupled receptors by human and murine chemokines.J Virol. 2004 Apr;78(7):3343-51. doi: 10.1128/jvi.78.7.3343-3351.2004. J Virol. 2004. PMID: 15016856 Free PMC article.
-
Acquired immunodeficiency syndrome-related malignancies in the era of highly active antiretroviral therapy.Int J Hematol. 2006 Jul;84(1):3-11. doi: 10.1532/IJH97.06088. Int J Hematol. 2006. PMID: 16867895 Review.
References
-
- Offermann MK. Kaposi's sarcoma and HHV-8. Trends Microbiol. 1996;4:419. - PubMed
-
- Nador RG, Cesarman E, Chadburn A, Dawson DB, Ansari MQ, Said J, Knowles DM. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi's sarcoma-associated herpes virus. Blood. 1996;88:645–656. - PubMed
-
- Arvanitakis L, Mesri EA, Nador RG, Said JW, Asch AS, Knowles DM, Cesarman E. Establishment and characterization of a body cavity-based lymphoma cell line (BC-3) harboring Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood. 1996;86:2708–2714. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

