PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate
- PMID: 9566978
- PMCID: PMC2132737
- DOI: 10.1083/jcb.141.3.805
PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate
Erratum in
- J Cell Biol 1998 Jun 1;141(5):1287
Abstract
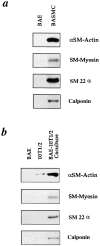
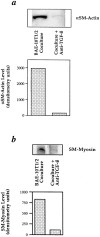
We aimed to determine if and how endothelial cells (EC) recruit precursors of smooth muscle cells and pericytes and induce their differentiation during vessel formation. Multipotent embryonic 10T1/2 cells were used as presumptive mural cell precursors. In an under-agarose coculture, EC induced migration of 10T1/2 cells via platelet-derived growth factor BB. 10T1/2 cells in coculture with EC changed from polygonal to spindle-shaped, reminiscent of smooth muscle cells in culture. Immunohistochemical and Western blot analyses were used to examine the expression of smooth muscle (SM)-specific markers in 10T1/2 cells cultured in the absence and presence of EC. SM-myosin, SM22alpha, and calponin proteins were undetectable in 10T1/2 cells cultured alone; however, expression of all three SM-specific proteins was significantly induced in 10T1/2 cells cocultured with EC. Treatment of 10T1/2 cells with TGF-beta induced phenotypic changes and changes in SM markers similar to those seen in the cocultures. Neutralization of TGF-beta in the cocultures blocked expression of the SM markers and the shape change. To assess the ability of 10T1/2 cells to contribute to the developing vessel wall in vivo, prelabeled 10T1/2 cells were grown in a collagen matrix and implanted subcutaneously into mice. The fluorescently marked cells became incorporated into the medial layer of developing vessels where they expressed SM markers. These in vitro and in vivo observations shed light on the cell-cell interactions that occur during vessel development, as well as in pathologies in which developmental processes are recapitulated.
Figures



Similar articles
-
Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact.Circ Res. 1999 Feb 19;84(3):298-305. doi: 10.1161/01.res.84.3.298. Circ Res. 1999. PMID: 10024303
-
TGF beta is required for the formation of capillary-like structures in three-dimensional cocultures of 10T1/2 and endothelial cells.Angiogenesis. 2001;4(1):11-20. doi: 10.1023/a:1016611824696. Angiogenesis. 2001. PMID: 11824373
-
Endothelial-mesenchymal interactions in vitro reveal molecular mechanisms of smooth muscle/pericyte differentiation.Stem Cells Dev. 2004 Oct;13(5):509-20. doi: 10.1089/scd.2004.13.509. Stem Cells Dev. 2004. PMID: 15588508
-
Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival.Dev Biol. 2003 Dec 1;264(1):275-88. doi: 10.1016/j.ydbio.2003.08.015. Dev Biol. 2003. PMID: 14623248
-
[Activation of latent TGF-beta. A required mechanism for vascular integrity].Pathol Biol (Paris). 1999 Apr;47(4):322-9. Pathol Biol (Paris). 1999. PMID: 10372400 Review. French.
Cited by
-
Vascular smooth muscle cell Smad4 gene is important for mouse vascular development.Arterioscler Thromb Vasc Biol. 2012 Sep;32(9):2171-7. doi: 10.1161/ATVBAHA.112.253872. Epub 2012 Jul 5. Arterioscler Thromb Vasc Biol. 2012. PMID: 22772757 Free PMC article.
-
Endothelial cell-pericyte interactions stimulate basement membrane matrix assembly: influence on vascular tube remodeling, maturation, and stabilization.Microsc Microanal. 2012 Feb;18(1):68-80. doi: 10.1017/S1431927611012402. Epub 2011 Dec 14. Microsc Microanal. 2012. PMID: 22166617 Free PMC article. Review.
-
Construction of vascular tissues with macro-porous nano-fibrous scaffolds and smooth muscle cells enriched from differentiated embryonic stem cells.PLoS One. 2012;7(4):e35580. doi: 10.1371/journal.pone.0035580. Epub 2012 Apr 24. PLoS One. 2012. PMID: 22545119 Free PMC article.
-
Heparan sulfate biosynthesis enzymes in embryonic stem cell biology.J Histochem Cytochem. 2012 Dec;60(12):943-9. doi: 10.1369/0022155412465090. Epub 2012 Oct 4. J Histochem Cytochem. 2012. PMID: 23042480 Free PMC article. Review.
-
Cell layer-electrospun mesh composites for coronary artery bypass grafts.J Biomed Mater Res A. 2016 Sep;104(9):2200-9. doi: 10.1002/jbm.a.35753. Epub 2016 May 4. J Biomed Mater Res A. 2016. PMID: 27101019 Free PMC article.
References
-
- Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117:1183–1198. - PubMed
-
- Applegate D, Feng W, Green RS, Taubman MB. Cloning and expression of a novel acidic calponin isoform from rat aortic vascular smooth muscle. J Biol Chem. 1994;269:10683–10690. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

