CAS/Crk coupling serves as a "molecular switch" for induction of cell migration
- PMID: 9472046
- PMCID: PMC2141747
- DOI: 10.1083/jcb.140.4.961
CAS/Crk coupling serves as a "molecular switch" for induction of cell migration
Abstract
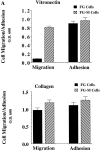
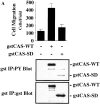
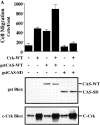
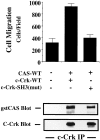
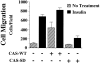
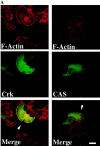
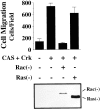
Carcinoma cells selected for their ability to migrate in vitro showed enhanced invasive properties in vivo. Associated with this induction of migration was the anchorage-dependent phosphorylation of p130CAS (Crk-associated substrate), leading to its coupling to the adaptor protein c-CrkII (Crk). In fact, expression of CAS or its adaptor protein partner Crk was sufficient to promote cell migration, and this depended on CAS tyrosine phosphorylation facilitating an SH2-mediated complex with Crk. Cytokine-stimulated cell migration was blocked by CAS lacking the Crk binding site or Crk containing a mutant SH2 domain. This migration response was characterized by CAS/Crk localization to membrane ruffles and blocked by the dominant-negative GTPase, Rac, but not Ras. Thus, CAS/Crk assembly serves as a "molecular switch" for the induction of cell migration and appears to contribute to the invasive property of tumors.
Figures













Similar articles
-
Insulin stimulates the tyrosine dephosphorylation of docking protein p130cas (Crk-associated substrate), promoting the switch of the adaptor protein crk from p130cas to newly phosphorylated insulin receptor substrate-1.Biochem J. 1998 Sep 15;334 ( Pt 3)(Pt 3):595-600. doi: 10.1042/bj3340595. Biochem J. 1998. PMID: 9729467 Free PMC article.
-
Regulation of cell contraction and membrane ruffling by distinct signals in migratory cells.J Cell Biol. 1999 Sep 6;146(5):1107-16. doi: 10.1083/jcb.146.5.1107. J Cell Biol. 1999. PMID: 10477763 Free PMC article.
-
Laminin-10/11 and fibronectin differentially regulate integrin-dependent Rho and Rac activation via p130(Cas)-CrkII-DOCK180 pathway.J Biol Chem. 2001 Jul 20;276(29):27090-7. doi: 10.1074/jbc.M102284200. Epub 2001 May 21. J Biol Chem. 2001. PMID: 11369773
-
Regulation of integrin-mediated cellular responses through assembly of a CAS/Crk scaffold.Biochim Biophys Acta. 2004 Jul 5;1692(2-3):63-76. doi: 10.1016/j.bbamcr.2004.03.006. Biochim Biophys Acta. 2004. PMID: 15246680 Review.
-
Physiological signals and oncogenesis mediated through Crk family adapter proteins.J Cell Physiol. 1998 Dec;177(4):535-52. doi: 10.1002/(SICI)1097-4652(199812)177:4<535::AID-JCP5>3.0.CO;2-E. J Cell Physiol. 1998. PMID: 10092207 Review.
Cited by
-
Involvement of NEDD9 in the invasion and migration of gastric cancer.Tumour Biol. 2015 May;36(5):3621-8. doi: 10.1007/s13277-014-2999-1. Epub 2015 Jan 12. Tumour Biol. 2015. PMID: 25577245
-
Individual Cas phosphorylation sites are dispensable for processive phosphorylation by Src and anchorage-independent cell growth.J Biol Chem. 2006 Jul 28;281(30):20689-20697. doi: 10.1074/jbc.M602311200. Epub 2006 May 17. J Biol Chem. 2006. PMID: 16707485 Free PMC article.
-
CEACAM engagement by human pathogens enhances cell adhesion and counteracts bacteria-induced detachment of epithelial cells.J Cell Biol. 2005 Aug 29;170(5):825-36. doi: 10.1083/jcb.200412151. Epub 2005 Aug 22. J Cell Biol. 2005. PMID: 16115956 Free PMC article.
-
Adaptor protein Crk is required for ephrin-B1-induced membrane ruffling and focal complex assembly of human aortic endothelial cells.Mol Biol Cell. 2002 Dec;13(12):4231-42. doi: 10.1091/mbc.e02-04-0181. Mol Biol Cell. 2002. PMID: 12475948 Free PMC article.
-
P130Cas-associated protein (p140Cap) as a new tyrosine-phosphorylated protein involved in cell spreading.Mol Biol Cell. 2004 Feb;15(2):787-800. doi: 10.1091/mbc.e03-09-0689. Epub 2003 Dec 2. Mol Biol Cell. 2004. PMID: 14657239 Free PMC article.
References
-
- Bar-Sagi D, Rotin D, Batzer A, Mandiyan V, Schlessinger J. SH3 domains direct cellular localization of signaling molecules. Cell. 1993;74:83–91. - PubMed
-
- Birge B, Fajardo J, Mayer B, Hanafusa H. Tyrosine-phosphorylated epidermal growth factor receptor and cellular p130 provide high affinity binding substrates to analyze Crk-phosphotyrosine-dependent interactions in vitro. J Biol Chem. 1992;267:10588–10595. - PubMed
-
- Cary LA, Chang JF, Guan J. Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J Cell Sci. 1996;109:1787–1794. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

