Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice
- PMID: 9463410
- PMCID: PMC2212142
- DOI: 10.1084/jem.187.4.601
Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice
Abstract
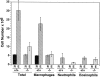
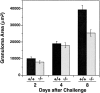
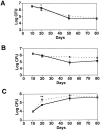
Monocyte chemoattractant protein 1 (MCP-1) is a CC chemokine that attracts monocytes, memory T lymphocytes, and natural killer cells. Because other chemokines have similar target cell specificities and because CCR2, a cloned MCP-1 receptor, binds other ligands, it has been uncertain whether MCP-1 plays a unique role in recruiting mononuclear cells in vivo. To address this question, we disrupted SCYA2 (the gene encoding MCP-1) and tested MCP-1-deficient mice in models of inflammation. Despite normal numbers of circulating leukocytes and resident macrophages, MCP-1(-/-) mice were specifically unable to recruit monocytes 72 h after intraperitoneal thioglycollate administration. Similarly, accumulation of F4/80+ monocytes in delayed-type hypersensitivity lesions was impaired, although the swelling response was normal. Development of secondary pulmonary granulomata in response to Schistosoma mansoni eggs was blunted in MCP-1(-/-) mice, as was expression of IL-4, IL-5, and interferon gamma in splenocytes. In contrast, MCP-1(-/-) mice were indistinguishable from wild-type mice in their ability to clear Mycobacterium tuberculosis. Our data indicate that MCP-1 is uniquely essential for monocyte recruitment in several inflammatory models in vivo and influences expression of cytokines related to T helper responses.
Figures

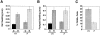
Similar articles
-
Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice.J Clin Invest. 1997 Nov 15;100(10):2552-61. doi: 10.1172/JCI119798. J Clin Invest. 1997. PMID: 9366570 Free PMC article.
-
Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis.J Exp Med. 2001 Mar 19;193(6):713-26. doi: 10.1084/jem.193.6.713. J Exp Med. 2001. PMID: 11257138 Free PMC article.
-
Role of monocyte chemoattractant protein-1 (MCP-1) in Th1 (mycobacterial) and Th2 (schistosomal) antigen-induced granuloma formation: relationship to local inflammation, Th cell expression, and IL-12 production.J Immunol. 1996 Nov 15;157(10):4602-8. J Immunol. 1996. PMID: 8906839
-
In vivo properties of monocyte chemoattractant protein-1.J Leukoc Biol. 1997 Nov;62(5):577-80. doi: 10.1002/jlb.62.5.577. J Leukoc Biol. 1997. PMID: 9365111 Review.
-
The production of monocyte chemoattractant protein-1 (MCP-1)/CCL2 in tumor microenvironments.Cytokine. 2017 Oct;98:71-78. doi: 10.1016/j.cyto.2017.02.001. Epub 2017 Feb 8. Cytokine. 2017. PMID: 28189389 Review.
Cited by
-
Preliminary proteomic analysis of mouse lung tissue treated with cyclophosphamide and Venetin-1.Sci Rep. 2024 Oct 23;14(1):25056. doi: 10.1038/s41598-024-76143-0. Sci Rep. 2024. PMID: 39443613 Free PMC article.
-
Effects of methylmercury contained in a diet mimicking the Wayana Amerindians contamination through fish consumption: mercury accumulation, metallothionein induction, gene expression variations, and role of the chemokine CCL2.Int J Mol Sci. 2012;13(6):7710-7738. doi: 10.3390/ijms13067710. Epub 2012 Jun 21. Int J Mol Sci. 2012. PMID: 22837723 Free PMC article.
-
Chronic photo-oxidative stress and subsequent MCP-1 activation as causative factors for age-related macular degeneration.J Cell Sci. 2012 May 15;125(Pt 10):2407-15. doi: 10.1242/jcs.097683. Epub 2012 Feb 22. J Cell Sci. 2012. PMID: 22357958 Free PMC article.
-
Studies in a Murine Granuloma Model of Instilled Carbon Nanotubes: Relevance to Sarcoidosis.Int J Mol Sci. 2021 Apr 2;22(7):3705. doi: 10.3390/ijms22073705. Int J Mol Sci. 2021. PMID: 33918196 Free PMC article. Review.
-
Imiquimod clears tumors in mice independent of adaptive immunity by converting pDCs into tumor-killing effector cells.J Clin Invest. 2012 Feb;122(2):575-85. doi: 10.1172/JCI61034. Epub 2012 Jan 17. J Clin Invest. 2012. PMID: 22251703 Free PMC article.
References
-
- Rollins BJ. Chemokines. Blood. 1997;90:909–928. - PubMed
-
- Valente AJ, Graves DT, Vialle-Valentin CE, Delgado R, Schwartz CJ. Purification of a monocyte chemotactic factor secreted by nonhuman primate vascular cells in culture. Biochemistry. 1988;27:4162–4168. - PubMed
-
- Allavena P, Bianchi G, Zhou D, van Damme J, Jilek P, Sozzani S, Mantovani A. Induction of natural killer cell migration by monocyte chemotactic protein–1, –2, and –3. Eur J Immunol. 1994;24:3233–3236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

