Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis
- PMID: 9435252
- PMCID: PMC18480
- DOI: 10.1073/pnas.95.2.681
Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis
Abstract
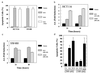
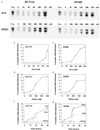
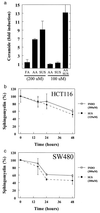
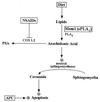
Nonsteroidal antiinflammatory drugs (NSAIDs) can inhibit colorectal tumorigenesis and are among the few agents known to be useful for the chemoprevention of neoplasia. Here, we show that the tumor suppressive effects of NSAIDs are not likely to be related to a reduction in prostaglandins but rather are due to the elevation of the prostaglandin precursor arachidonic acid (AA). NSAID treatment of colon tumor cells results in a dramatic increase in AA that in turn stimulates the conversion of sphingomyelin to ceramide, a known mediator of apoptosis. These results have significant implications for understanding and improving colon cancer chemoprevention.
Figures

Similar articles
-
COX-2, NSAIDs and human neoplasia. Part I: Colorectal neoplasms.In Vivo. 2002 Nov-Dec;16(6):501-9. In Vivo. 2002. PMID: 12494894 Review.
-
The 15-lipoxygenase-1 product 13-S-hydroxyoctadecadienoic acid down-regulates PPAR-delta to induce apoptosis in colorectal cancer cells.Proc Natl Acad Sci U S A. 2003 Aug 19;100(17):9968-73. doi: 10.1073/pnas.1631086100. Epub 2003 Aug 8. Proc Natl Acad Sci U S A. 2003. PMID: 12909723 Free PMC article.
-
Cyclooxygenase expression is not required for release of arachidonic acid from cells by some nonsteroidal anti-inflammatory drugs and cancer preventive agents.BMC Pharmacol. 2006 Mar 29;6:7. doi: 10.1186/1471-2210-6-7. BMC Pharmacol. 2006. PMID: 16571133 Free PMC article.
-
The role of arachidonic acid regulatory enzymes in colorectal disease.Dis Colon Rectum. 2005 Jul;48(7):1471-83. doi: 10.1007/s10350-005-0015-y. Dis Colon Rectum. 2005. PMID: 15868226 Review.
-
Nonsteroidal anti-inflammatory drugs and oxidative stress in cancer cells.Histol Histopathol. 2007 Apr;22(4):437-42. doi: 10.14670/HH-22.437. Histol Histopathol. 2007. PMID: 17290354 Review.
Cited by
-
The expression of phospholipase A2 group X is inversely associated with metastasis in colorectal cancer.Oncol Lett. 2013 Feb;5(2):533-538. doi: 10.3892/ol.2012.1067. Epub 2012 Dec 10. Oncol Lett. 2013. PMID: 23420493 Free PMC article.
-
Systems Pharmacology Dissection of the Integrated Treatment for Cardiovascular and Gastrointestinal Disorders by Traditional Chinese Medicine.Sci Rep. 2016 Sep 6;6:32400. doi: 10.1038/srep32400. Sci Rep. 2016. PMID: 27597117 Free PMC article.
-
Impact of group IVA cytosolic phospholipase A2 gene polymorphisms on phenotypic features of patients with familial adenomatous polyposis.Int J Colorectal Dis. 2010 Mar;25(3):293-301. doi: 10.1007/s00384-009-0808-x. Epub 2009 Oct 1. Int J Colorectal Dis. 2010. PMID: 19795129
-
Ion channel inhibitors block caspase activation by mechanisms other than restoring intracellular potassium concentration.Cell Death Dis. 2011 Jan 13;2(1):e113. doi: 10.1038/cddis.2010.93. Cell Death Dis. 2011. PMID: 21368885 Free PMC article.
-
Cytochrome p450 epoxygenase metabolism of arachidonic acid inhibits apoptosis.Mol Cell Biol. 2001 Sep;21(18):6322-31. doi: 10.1128/MCB.21.18.6322-6331.2001. Mol Cell Biol. 2001. PMID: 11509673 Free PMC article.
References
-
- Giardiello F M. Gastroenterol Clin North Am. 1996;25:349–362. - PubMed
-
- Beazer-Barclay Y, Levy D B, Moser A M, Dove W F, Hamilton S R, Vogelstein B, Kinzler K W. Carcinogenesis. 1996;17:1757–1760. - PubMed
-
- Boolbol S K, Dannenberg A J, Chadurn A, Martucci C, Guo X, Ramonetti J T, Abreu-Goris M, Newmark H L, Lipkin M L, DeCosse J J, Bertagnolli M M. Cancer Res. 1996;56:2556–2560. - PubMed
-
- Jacoby R F, Marshall D J, Newton M A, Novakovic K, Tutsch K, Cole C E, Lubet R A, Kelloff G J, Verma A, Moser A R, Dove W F. Cancer Res. 1996;56:710–714. - PubMed
-
- Thun M J. Gastroenterol Clin North Am. 1996;25:333–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

