Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant
- PMID: 9391135
- PMCID: PMC28415
- DOI: 10.1073/pnas.94.25.13961
Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant
Abstract
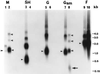
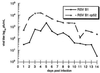
A live, cold-passaged (cp) candidate vaccine virus, designated respiratory syncytial virus (RSV) B1 cp-52/2B5 (cp-52), replicated efficiently in Vero cells, but was found to be overattenuated for RSV-seronegative infants and children. Sequence analysis of reverse-transcription-PCR-amplified fragments of this mutant revealed a large deletion spanning most of the coding sequences for the small hydrophobic (SH) and attachment (G) proteins. Northern blot analysis of cp-52 detected multiple unique read-through mRNAs containing SH and G sequences, consistent with a deletion mutation spanning the SH:G gene junction. Immunological studies confirmed that an intact G glycoprotein was not produced by the cp-52 virus. Nonetheless, cp-52 was infectious and replicated to high titer in tissue culture despite the absence of the viral surface SH and G glycoproteins. Thus, our characterization of this negative-strand RNA virus identified a novel replication-competent deletion mutant lacking two of its three surface glycoproteins. The requirement of SH and G for efficient replication in vivo suggests that selective deletion of one or both of these RSV genes may provide an alternative or additive strategy for developing an optimally attenuated vaccine candidate.
Figures

Similar articles
-
Replacement of the F and G proteins of respiratory syncytial virus (RSV) subgroup A with those of subgroup B generates chimeric live attenuated RSV subgroup B vaccine candidates.J Virol. 1999 Dec;73(12):9773-80. doi: 10.1128/JVI.73.12.9773-9780.1999. J Virol. 1999. PMID: 10559287 Free PMC article.
-
Addition of a missense mutation present in the L gene of respiratory syncytial virus (RSV) cpts530/1030 to RSV vaccine candidate cpts248/404 increases its attenuation and temperature sensitivity.J Virol. 1999 Feb;73(2):871-7. doi: 10.1128/JVI.73.2.871-877.1999. J Virol. 1999. PMID: 9882287 Free PMC article. Clinical Trial.
-
Recombinant respiratory syncytial virus from which the entire SH gene has been deleted grows efficiently in cell culture and exhibits site-specific attenuation in the respiratory tract of the mouse.J Virol. 1997 Dec;71(12):8973-82. doi: 10.1128/JVI.71.12.8973-8982.1997. J Virol. 1997. PMID: 9371553 Free PMC article.
-
Evaluation of the Safety and Immune Efficacy of Recombinant Human Respiratory Syncytial Virus Strain Long Live Attenuated Vaccine Candidates.Virol Sin. 2021 Aug;36(4):706-720. doi: 10.1007/s12250-021-00345-3. Epub 2021 Feb 9. Virol Sin. 2021. PMID: 33559831 Free PMC article. Review.
-
Respiratory syncytial virus infection in older persons.Vaccine. 1998 Nov;16(18):1775-8. doi: 10.1016/s0264-410x(98)00142-x. Vaccine. 1998. PMID: 9778756 Review.
Cited by
-
Structure-guided design of small-molecule therapeutics against RSV disease.Expert Opin Drug Discov. 2016;11(6):543-556. doi: 10.1517/17460441.2016.1174212. Epub 2016 Apr 21. Expert Opin Drug Discov. 2016. PMID: 27046051 Free PMC article.
-
Respiratory syncytial virus fusion protein-induced toll-like receptor 4 (TLR4) signaling is inhibited by the TLR4 antagonists Rhodobacter sphaeroides lipopolysaccharide and eritoran (E5564) and requires direct interaction with MD-2.mBio. 2012 Aug 7;3(4):e00218-12. doi: 10.1128/mBio.00218-12. Print 2012. mBio. 2012. PMID: 22872782 Free PMC article.
-
Respiratory syncytial virus G and/or SH protein alters Th1 cytokines, natural killer cells, and neutrophils responding to pulmonary infection in BALB/c mice.J Virol. 1999 Sep;73(9):7099-107. doi: 10.1128/JVI.73.9.7099-7107.1999. J Virol. 1999. PMID: 10438795 Free PMC article.
-
Detection and activation of HIV broadly neutralizing antibody precursor B cells using anti-idiotypes.J Exp Med. 2019 Oct 7;216(10):2331-2347. doi: 10.1084/jem.20190164. Epub 2019 Jul 25. J Exp Med. 2019. PMID: 31345930 Free PMC article. Clinical Trial.
-
Determining Immune and miRNA Biomarkers Related to Respiratory Syncytial Virus (RSV) Vaccine Types.Front Immunol. 2019 Oct 9;10:2323. doi: 10.3389/fimmu.2019.02323. eCollection 2019. Front Immunol. 2019. PMID: 31649663 Free PMC article.
References
-
- Collins P L, McIntosh K, Chanock R M. In: Fields Virology. Fields B N, editor. New York: Raven; 1996. pp. 1313–1351.
-
- Anderson L J, Parker R A, Strikas R L. J Infect Dis. 1990;161:640–646. - PubMed
-
- Committee on Issues and Priorities for New Vaccine Development. New Vaccine Development: Establishing Priorities. National Academy Press, Washington, DC: Institute of Medicine; 1985. p. 342.
-
- Murphy B R, Hall S L, Kulkarni A B, Crowe J E, Collins P L, Connors M, Karron R A, Chanock R M. Virus Res. 1994;32:13–36. - PubMed
-
- Taylor C E, Morrow S, Scott M, Young B, Toms G L. Lancet. 1989;1:777–778. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

