Quantitative analysis reveals expansion of human hematopoietic repopulating cells after short-term ex vivo culture
- PMID: 9254660
- PMCID: PMC2199026
- DOI: 10.1084/jem.186.4.619
Quantitative analysis reveals expansion of human hematopoietic repopulating cells after short-term ex vivo culture
Abstract
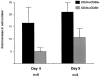
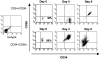
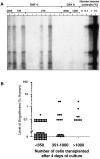
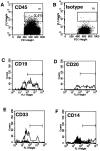
Ex vivo culture of human hematopoietic cells is a crucial component of many therapeutic applications. Although current culture conditions have been optimized using quantitative in vitro progenitor assays, knowledge of the conditions that permit maintenance of primitive human repopulating cells is lacking. We report that primitive human cells capable of repopulating nonobese diabetic (NOD)/severe combined immunodeficiency (SCID) mice (SCID-repopulating cells; SRC) can be maintained and/or modestly increased after culture of CD34+CD38- cord blood cells in serum-free conditions. Quantitative analysis demonstrated a 4- and 10-fold increase in the number of CD34+CD38- cells and colony-forming cells, respectively, as well as a 2- to 4-fold increase in SRC after 4 d of culture. However, after 9 d of culture, all SRC were lost, despite further increases in total cells, CFC content, and CD34+ cells. These studies indicate that caution must be exercised in extending the duration of ex vivo cultures used for transplantation, and demonstrate the importance of the SRC assay in the development of culture conditions that support primitive cells.
Figures
Similar articles
-
Differential maintenance of primitive human SCID-repopulating cells, clonogenic progenitors, and long-term culture-initiating cells after incubation on human bone marrow stromal cells.Blood. 1997 Jul 15;90(2):641-50. Blood. 1997. PMID: 9226164
-
Dissociation between stem cell phenotype and NOD/SCID repopulating activity in human peripheral blood CD34(+) cells after ex vivo expansion.Exp Hematol. 2001 Dec;29(12):1465-73. doi: 10.1016/s0301-472x(01)00750-0. Exp Hematol. 2001. PMID: 11750106
-
Expansion of human cord blood CD34(+)CD38(-) cells in ex vivo culture during retroviral transduction without a corresponding increase in SCID repopulating cell (SRC) frequency: dissociation of SRC phenotype and function.Blood. 2000 Jan 1;95(1):102-10. Blood. 2000. PMID: 10607692
-
Absence of CD34 on some human SCID-repopulating cells.Ann N Y Acad Sci. 1999 Apr 30;872:211-7; discussion 217-9. doi: 10.1111/j.1749-6632.1999.tb08466.x. Ann N Y Acad Sci. 1999. PMID: 10372124 Review.
-
Integrative molecular and developmental biology of adult stem cells.Biol Cell. 2003 Dec;95(9):563-78. doi: 10.1016/j.biolcel.2003.10.001. Biol Cell. 2003. PMID: 14720459 Review.
Cited by
-
Ex Vivo Expansion of Hematopoietic Stem Cells for Therapeutic Purposes: Lessons from Development and the Niche.Cells. 2019 Feb 18;8(2):169. doi: 10.3390/cells8020169. Cells. 2019. PMID: 30781676 Free PMC article. Review.
-
Expression of CD45 in non-hematopoietic cells: implications in regenerative medicine and disease management.Regen Med. 2024;19(7-8):407-419. doi: 10.1080/17460751.2024.2378627. Epub 2024 Jul 26. Regen Med. 2024. PMID: 39058408 Review.
-
IL3 Has a Detrimental Effect on Hematopoietic Stem Cell Self-Renewal in Transplantation Settings.Int J Mol Sci. 2022 Oct 22;23(21):12736. doi: 10.3390/ijms232112736. Int J Mol Sci. 2022. PMID: 36361533 Free PMC article.
-
Optimizing autologous cell grafts to improve stem cell gene therapy.Exp Hematol. 2016 Jul;44(7):528-39. doi: 10.1016/j.exphem.2016.04.007. Epub 2016 Apr 19. Exp Hematol. 2016. PMID: 27106799 Free PMC article. Review.
-
Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells.Proc Natl Acad Sci U S A. 2006 Aug 1;103(31):11707-12. doi: 10.1073/pnas.0603806103. Epub 2006 Jul 20. Proc Natl Acad Sci U S A. 2006. PMID: 16857736 Free PMC article.
References
-
- Moore MA. Expansion of myeloid stem cells in culture. Semin Hematol. 1995;32:183–200. - PubMed
-
- Williams DA. Ex vivo expansion of hematopoietic stem and progenitor cells–robbing Peter to pay Paul? . Blood. 1993;81:3169–3172. - PubMed
-
- Brugger W, Heimfeld S, Berenson RJ, Mertelsmann R, Kanz L. Reconstitution of hematopoiesis after high-dose chemotherapy by autologous progenitor cells generated ex vivo. N Engl J Med. 1995;333:283–287. - PubMed
-
- Chang J, Coutinho L, Morgenstern G, Scarffe JH, Deakin D, Harrison C, Testa NG, Dexter TM. Reconstitution of haemopoietic system with autologous marrow taken during relapse of acute myeloblastic leukaemia and grown in long-term culture. Lancet. 1986;1:294–295. - PubMed
-
- Barnett MJ, Eaves CJ, Phillips GL, Gascoyne RD, Hogge DE, Horsman DE, Humphries RK, Klingemann HG, Lansdorp PM, Nantel SH. Autografting with cultured marrow in chronic myeloid leukemia: results of a pilot study. Blood. 1994;84:724–732. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

