The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts
- PMID: 9182672
- PMCID: PMC2132529
- DOI: 10.1083/jcb.137.6.1421
The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts
Abstract
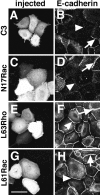
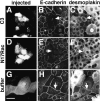
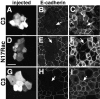
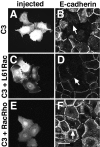
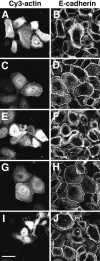
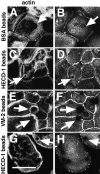
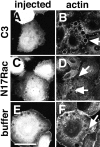
Cadherins are calcium-dependent cell-cell adhesion molecules that require the interaction of the cytoplasmic tail with the actin cytoskeleton for adhesive activity. Because of the functional relationship between cadherin receptors and actin filament organization, we investigated whether members of the Rho family of small GTPases are necessary for cadherin adhesion. In fibroblasts, the Rho family members Rho and Rac regulate actin polymerization to produce stress fibers and lamellipodia, respectively. In epithelial cells, we demonstrate that Rho and Rac are required for the establishment of cadherin-mediated cell-cell adhesion and the actin reorganization necessary to stabilize the receptors at sites of intercellular junctions. Blocking endogenous Rho or Rac selectively removed cadherin complexes from junctions induced for up to 3 h, while desmosomes were not perturbed. In addition, withdrawal of cadherins from intercellular junctions temporally precedes the removal of CD44 and integrins, other microfilament-associated receptors. Our data showed that the concerted action of Rho and Rac modulate the establishment of cadherin adhesion: a constitutively active form of Rac was not sufficient to stabilize cadherindependent cell-cell contacts when endogenous Rho was inhibited. Upon induction of calcium-dependent intercellular adhesion, there was a rapid accumulation of actin at sites of cell-cell contacts, which was prevented by blocking cadherin function, Rho or Rac activity. However, if cadherin complexes are clustered by specific antibodies attached to beads, actin recruitment to the receptors was perturbed by inhibiting Rac but not Rho. Our results provide new insights into the role of the small GTPases in the cadherin-dependent cell- cell contact formation and the remodelling of actin filaments in epithelial cells.
Figures

Similar articles
-
Regulation of cadherin function by Rho and Rac: modulation by junction maturation and cellular context.Mol Biol Cell. 1999 Jan;10(1):9-22. doi: 10.1091/mbc.10.1.9. Mol Biol Cell. 1999. PMID: 9880323 Free PMC article.
-
Role of actin polymerization and adhesion to extracellular matrix in Rac- and Rho-induced cytoskeletal reorganization.J Cell Biol. 1997 Aug 25;138(4):913-26. doi: 10.1083/jcb.138.4.913. J Cell Biol. 1997. PMID: 9265656 Free PMC article.
-
Rac-WAVE-mediated actin reorganization is required for organization and maintenance of cell-cell adhesion.J Cell Sci. 2007 Jan 1;120(Pt 1):86-100. doi: 10.1242/jcs.03311. Epub 2006 Dec 12. J Cell Sci. 2007. PMID: 17164293
-
Epithelial cell shape: cadherins and small GTPases.Exp Cell Res. 2000 Nov 25;261(1):83-90. doi: 10.1006/excr.2000.5050. Exp Cell Res. 2000. PMID: 11082278 Review.
-
Coordinating Rho and Rac: the regulation of Rho GTPase signaling and cadherin junctions.Prog Mol Biol Transl Sci. 2013;116:49-68. doi: 10.1016/B978-0-12-394311-8.00003-0. Prog Mol Biol Transl Sci. 2013. PMID: 23481190 Review.
Cited by
-
Immunohistological study of small Rho GTPases and β-catenin during regeneration of the rat submandibular gland.J Mol Histol. 2012 Dec;43(6):751-9. doi: 10.1007/s10735-012-9437-8. Epub 2012 Jul 17. J Mol Histol. 2012. PMID: 22802017
-
From mechanical force to RhoA activation.Biochemistry. 2012 Sep 25;51(38):7420-32. doi: 10.1021/bi300758e. Epub 2012 Sep 10. Biochemistry. 2012. PMID: 22931484 Free PMC article. Review.
-
Follicular thyroid tumors with the PAX8-PPARgamma1 rearrangement display characteristic genetic alterations.Am J Pathol. 2005 Jul;167(1):223-31. doi: 10.1016/s0002-9440(10)62967-7. Am J Pathol. 2005. PMID: 15972966 Free PMC article.
-
Primary mouse renal tubular epithelial cells have variable injury tolerance to ischemic and chemical mediators of oxidative stress.Oxid Med Cell Longev. 2008 Oct-Dec;1(1):33-8. doi: 10.4161/oxim.1.1.6491. Oxid Med Cell Longev. 2008. PMID: 19794906 Free PMC article.
-
Microtubules: Evolving roles and critical cellular interactions.Exp Biol Med (Maywood). 2019 Nov;244(15):1240-1254. doi: 10.1177/1535370219867296. Epub 2019 Aug 6. Exp Biol Med (Maywood). 2019. PMID: 31387376 Free PMC article. Review.
References
-
- Amagai M, Fujimori T, Masunaga T, Shimizu H, Nishikawa T, Shimizu N, Takeichi M, Hashimoto T. Delayed assembly of desmosomes in keratinocytes with disrupted classic cadherin-mediated cell adhesion by a dominant negative mutant. J Investig Dermatol. 1995;104:27–32. - PubMed
-
- Behrens J, Vakaet L, Winterhager E, Van Roy F, Mareel MM, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/β-catenin complex in cells transformed with a temperature-sensitive v-srcgene. J Cell Biol. 1993;120:757–766. - PMC - PubMed
-
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedel R, Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature (Lond) 1996;382:638–642. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

