Evaluation of four KCNMA1 channelopathy variants on BK channel current under CaV1.2 activation
- PMID: 39217512
- PMCID: PMC11370921
- DOI: 10.1080/19336950.2024.2396346
Evaluation of four KCNMA1 channelopathy variants on BK channel current under CaV1.2 activation
Abstract
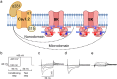
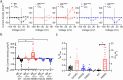
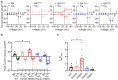
Variants in KCNMA1, encoding the voltage- and calcium-activated K+ (BK) channel, are associated with human neurological disease. The effects of gain-of-function (GOF) and loss-of-function (LOF) variants have been predominantly studied on BK channel currents evoked under steady-state voltage and Ca2+ conditions. However, in their physiological context, BK channels exist in partnership with voltage-gated Ca2+ channels and respond to dynamic changes in intracellular Ca2+ (Ca2+i). In this study, an L-type voltage-gated Ca2+ channel present in the brain, CaV1.2, was co-expressed with wild type and mutant BK channels containing GOF (D434G, N999S) and LOF (H444Q, D965V) patient-associated variants in HEK-293T cells. Whole-cell BK currents were recorded under CaV1.2 activation using buffering conditions that restrict Ca2+i to nano- or micro-domains. Both conditions permitted wild type BK current activation in response to CaV1.2 Ca2+ influx, but differences in behavior between wild type and mutant BK channels were reduced compared to prior studies in clamped Ca2+i. Only the N999S mutation produced an increase in BK current in both micro- and nano-domains using square voltage commands and was also detectable in BK current evoked by a neuronal action potential within a microdomain. These data corroborate the GOF effect of N999S on BK channel activity under dynamic voltage and Ca2+ stimuli, consistent with its pathogenicity in neurological disease. However, the patient-associated mutations D434G, H444Q, and D965V did not exhibit significant effects on BK current under CaV1.2-mediated Ca2+ influx, in contrast with prior steady-state protocols. These results demonstrate a differential potential for KCNMA1 variant pathogenicity compared under diverse voltage and Ca2+ conditions.
Keywords: CACNA1C; CaV1.2; KCa1.1; calcium-activated potassium channel; channelopathy; voltage-gated calcium channels.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

Similar articles
-
Comparative gain-of-function effects of the KCNMA1-N999S mutation on human BK channel properties.J Neurophysiol. 2020 Feb 1;123(2):560-570. doi: 10.1152/jn.00626.2019. Epub 2019 Dec 18. J Neurophysiol. 2020. PMID: 31851553 Free PMC article.
-
BK channel properties correlate with neurobehavioral severity in three KCNMA1-linked channelopathy mouse models.Elife. 2022 Jul 12;11:e77953. doi: 10.7554/eLife.77953. Elife. 2022. PMID: 35819138 Free PMC article.
-
Disease-associated KCNMA1 variants decrease circadian clock robustness in channelopathy mouse models.J Gen Physiol. 2023 Nov 6;155(11):e202313357. doi: 10.1085/jgp.202313357. Epub 2023 Sep 20. J Gen Physiol. 2023. PMID: 37728576 Free PMC article.
-
An emerging spectrum of variants and clinical features in KCNMA1-linked channelopathy.Channels (Austin). 2021 Dec;15(1):447-464. doi: 10.1080/19336950.2021.1938852. Channels (Austin). 2021. PMID: 34224328 Free PMC article. Review.
-
KCNMA1-linked channelopathy.J Gen Physiol. 2019 Oct 7;151(10):1173-1189. doi: 10.1085/jgp.201912457. Epub 2019 Aug 19. J Gen Physiol. 2019. PMID: 31427379 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
