Heterogeneity of endothelial VE-PTP downstream polarization, Tie2 activation, junctional claudin-5, and permeability in the aorta and vena cava
- PMID: 38032480
- PMCID: PMC10774230
- DOI: 10.1007/s00441-023-03844-9
Heterogeneity of endothelial VE-PTP downstream polarization, Tie2 activation, junctional claudin-5, and permeability in the aorta and vena cava
Abstract
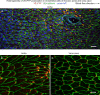
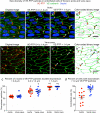
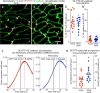
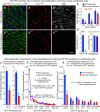
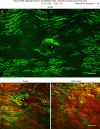
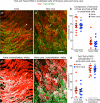
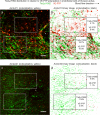
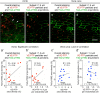
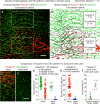
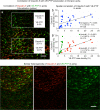
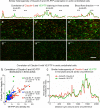
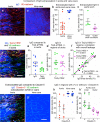
Endothelial cells of mammalian blood vessels have multiple levels of heterogeneity along the vascular tree and among different organs. Further heterogeneity results from blood flow turbulence and variations in shear stress. In the aorta, vascular endothelial protein tyrosine phosphatase (VE-PTP), which dephosphorylates tyrosine kinase receptor Tie2 in the plasma membrane, undergoes downstream polarization and endocytosis in endothelial cells exposed to laminar flow and high shear stress. VE-PTP sequestration promotes Tie2 phosphorylation at tyrosine992 and endothelial barrier tightening. The present study characterized the heterogeneity of VE-PTP polarization, Tie2-pY992 and total Tie2, and claudin-5 in anatomically defined regions of endothelial cells in the mouse descending thoracic aorta, where laminar flow is variable and IgG extravasation is patchy. We discovered that VE-PTP and Tie2-pY992 had mosaic patterns, unlike the uniform distribution of total Tie2. Claudin-5 at tight junctions also had a mosaic pattern, whereas VE-cadherin at adherens junctions bordered all endothelial cells. Importantly, the amounts of Tie2-pY992 and claudin-5 in aortic endothelial cells correlated with downstream polarization of VE-PTP. VE-PTP and Tie2-pY992 also had mosaic patterns in the vena cava, but claudin-5 was nearly absent and extravasated IgG was ubiquitous. Correlation of Tie2-pY992 and claudin-5 with VE-PTP polarization supports their collective interaction in the regulation of endothelial barrier function in the aorta, yet differences between the aorta and vena cava indicate additional flow-related determinants of permeability. Together, the results highlight new levels of endothelial cell functional mosaicism in the aorta and vena cava, where blood flow dynamics are well known to be heterogeneous.
Keywords: Adherens junctions; Blood flow; C57BL/6 mice; Endothelial barrier function; Immunohistochemistry; Receptor-type protein tyrosine phosphatase beta (PTPRB); Shear stress; Tight junctions; Vascular permeability.
© 2023. The Author(s).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

Similar articles
-
Shear stress control of vascular leaks and atheromas through Tie2 activation by VE-PTP sequestration.EMBO Mol Med. 2023 Apr 11;15(4):e16128. doi: 10.15252/emmm.202216128. Epub 2023 Feb 6. EMBO Mol Med. 2023. PMID: 36740996 Free PMC article.
-
Pyk2 phosphorylation of VE-PTP downstream of STIM1-induced Ca2+ entry regulates disassembly of adherens junctions.Am J Physiol Lung Cell Mol Physiol. 2017 Jun 1;312(6):L1003-L1017. doi: 10.1152/ajplung.00008.2017. Epub 2017 Apr 6. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28385807 Free PMC article.
-
Vascular Endothelial Receptor Tyrosine Phosphatase: Identification of Novel Substrates Related to Junctions and a Ternary Complex with EPHB4 and TIE2.Mol Cell Proteomics. 2019 Oct;18(10):2058-2077. doi: 10.1074/mcp.RA119.001716. Epub 2019 Aug 19. Mol Cell Proteomics. 2019. PMID: 31427368 Free PMC article.
-
Vascular Endothelial Protein Tyrosine Phosphatase Regulates Endothelial Function.Physiology (Bethesda). 2021 Mar 1;36(2):84-93. doi: 10.1152/physiol.00026.2020. Physiology (Bethesda). 2021. PMID: 33595386 Review.
-
Dynamic Regulation of Vascular Permeability by Vascular Endothelial Cadherin-Mediated Endothelial Cell-Cell Junctions.J Nippon Med Sch. 2017;84(4):148-159. doi: 10.1272/jnms.84.148. J Nippon Med Sch. 2017. PMID: 28978894 Review.
References
-
- Augustin HG, Koh GY (2017) Organotypic vasculature: From descriptive heterogeneity to functional pathophysiology. Science 357:eaal2379 - PubMed
-
- Baumer S, Keller L, Holtmann A, Funke R, August B, Gamp A, Wolburg H, Wolburg-Buchholz K, Deutsch U, Vestweber D. Vascular endothelial cell-specific phosphotyrosine phosphatase (VE-PTP) activity is required for blood vessel development. Blood. 2006;107:4754–4762. doi: 10.1182/blood-2006-01-0141. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

