Combinatorial blockade for cancer immunotherapy: targeting emerging immune checkpoint receptors
- PMID: 37928556
- PMCID: PMC10620683
- DOI: 10.3389/fimmu.2023.1264327
Combinatorial blockade for cancer immunotherapy: targeting emerging immune checkpoint receptors
Abstract
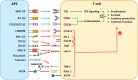
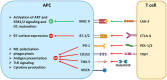
The differentiation, survival, and effector function of tumor-specific CD8+ cytotoxic T cells lie at the center of antitumor immunity. Due to the lack of proper costimulation and the abundant immunosuppressive mechanisms, tumor-specific T cells show a lack of persistence and exhausted and dysfunctional phenotypes. Multiple coinhibitory receptors, such as PD-1, CTLA-4, VISTA, TIGIT, TIM-3, and LAG-3, contribute to dysfunctional CTLs and failed antitumor immunity. These coinhibitory receptors are collectively called immune checkpoint receptors (ICRs). Immune checkpoint inhibitors (ICIs) targeting these ICRs have become the cornerstone for cancer immunotherapy as they have established new clinical paradigms for an expanding range of previously untreatable cancers. Given the nonredundant yet convergent molecular pathways mediated by various ICRs, combinatorial immunotherapies are being tested to bring synergistic benefits to patients. In this review, we summarize the mechanisms of several emerging ICRs, including VISTA, TIGIT, TIM-3, and LAG-3, and the preclinical and clinical data supporting combinatorial strategies to improve existing ICI therapies.
Keywords: CTLA-4; LAG3; PD-1; TIGIT; TIM3; VISTA; combinatorial immunotherapies; immune checkpoint inhibitors.
Copyright © 2023 Roy, Gilmour, Patnaik and Wang.
Conflict of interest statement
LW is an inventor involved with the commercial development of VISTA with ImmuNext Inc Corporation Lebanon, NH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
High co-expression of immune checkpoint receptors PD-1, CTLA-4, LAG-3, TIM-3, and TIGIT on tumor-infiltrating lymphocytes in early-stage breast cancer.World J Surg Oncol. 2022 Oct 21;20(1):349. doi: 10.1186/s12957-022-02810-z. World J Surg Oncol. 2022. PMID: 36271406 Free PMC article.
-
Antibodies Against Immune Checkpoint Molecules Restore Functions of Tumor-Infiltrating T Cells in Hepatocellular Carcinomas.Gastroenterology. 2017 Oct;153(4):1107-1119.e10. doi: 10.1053/j.gastro.2017.06.017. Epub 2017 Jun 23. Gastroenterology. 2017. PMID: 28648905
-
Targeting LAG-3, TIM-3, and TIGIT for cancer immunotherapy.J Hematol Oncol. 2023 Sep 5;16(1):101. doi: 10.1186/s13045-023-01499-1. J Hematol Oncol. 2023. PMID: 37670328 Free PMC article. Review.
-
A Comprehensive Analysis of Key Immune Checkpoint Receptors on Tumor-Infiltrating T Cells From Multiple Types of Cancer.Front Oncol. 2019 Oct 25;9:1066. doi: 10.3389/fonc.2019.01066. eCollection 2019. Front Oncol. 2019. PMID: 31709176 Free PMC article.
-
TIGIT in cancer immunotherapy.J Immunother Cancer. 2020 Sep;8(2):e000957. doi: 10.1136/jitc-2020-000957. J Immunother Cancer. 2020. PMID: 32900861 Free PMC article. Review.
Cited by
-
Immune restoration by TIGIT blockade is insufficient to control chronic SIV infection.J Virol. 2024 Jun 13;98(6):e0027324. doi: 10.1128/jvi.00273-24. Epub 2024 May 22. J Virol. 2024. PMID: 38775481 Free PMC article.
-
TIM-3, LAG-3, or 2B4 gene disruptions increase the anti-tumor response of engineered T cells.Front Immunol. 2024 Feb 29;15:1315283. doi: 10.3389/fimmu.2024.1315283. eCollection 2024. Front Immunol. 2024. PMID: 38510235 Free PMC article.
-
Mismatch repair-proficient tumor footprints in the sands of immune desert: mechanistic constraints and precision platforms.Front Immunol. 2024 Jul 19;15:1414376. doi: 10.3389/fimmu.2024.1414376. eCollection 2024. Front Immunol. 2024. PMID: 39100682 Free PMC article. Review.
-
Cutting-Edge Therapies for Lung Cancer.Cells. 2024 Mar 1;13(5):436. doi: 10.3390/cells13050436. Cells. 2024. PMID: 38474400 Free PMC article. Review.
-
Nanoparticles targeting immune checkpoint protein VISTA induce potent antitumor immunity.J Immunother Cancer. 2024 Aug 28;12(8):e008977. doi: 10.1136/jitc-2024-008977. J Immunother Cancer. 2024. PMID: 39209454 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

