Mental Health and Psychosocial Quality-of-Life Burden Among Patients With Vitiligo: Findings From the Global VALIANT Study
- PMID: 37647073
- PMCID: PMC10469285
- DOI: 10.1001/jamadermatol.2023.2787
Mental Health and Psychosocial Quality-of-Life Burden Among Patients With Vitiligo: Findings From the Global VALIANT Study
Erratum in
-
Error in Supplement 1.JAMA Dermatol. 2024 Jan 1;160(1):118. doi: 10.1001/jamadermatol.2023.4958. JAMA Dermatol. 2024. PMID: 37991742 Free PMC article. No abstract available.
Abstract
Importance: Patients with vitiligo often have impaired quality of life (QOL) and experience substantial psychosocial burden.
Objective: To explore the global association of vitiligo with QOL and mental health from the patient perspective.
Design, setting, and participants: This qualitative study of the cross-sectional population-based Vitiligo and Life Impact Among International Communities (VALIANT) study was conducted from May 6, 2021, to June 21, 2021. Potential participants for this qualitative study were recruited from an online panel in 17 countries. Of 5859 surveyed adults (aged ≥18 years) who reported a vitiligo diagnosis, 3919 (66.9%) completed the survey, and 3541 (60.4%) were included in the analysis.
Exposures: Patients were asked questions regarding their emotional well-being, including QOL and mental health.
Main outcomes and measures: Reported analyses are descriptive and hypothesis generating. Vitiligo Impact Patient scale (VIPs) scores ranged from 0 to 60, with higher scores indicating more psychosocial burden.
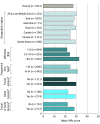
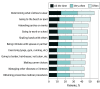
Results: The median age of the 3541 patients was 38 years (range, 18-95 years), and 1933 (54.6%) were male; 1602 patients (45.2%) had more than 5% affected body surface area (BSA; Self-Assessment Vitiligo Extent Score assessed), and 1445 patients (40.8%) had Fitzpatrick skin types IV to VI (ie, darker skin). The mean (SD) global short-form VIPs score was 27.3 (15.6) overall; patients from India (mean [SD], 40.2 [14.1]) reported the highest scores (ie, most burden). The QOL burden according to the scale was profound for patients with more than 5% affected BSA (mean [SD] score, 32.6 [14.2]), darker skin (mean [SD] score, 31.2 [15.6]), and lesions on the face (mean [SD] score, 30.0 [14.9]) or hands (mean [SD], 29.2 [15.2]). At least 40% of patients globally reported that vitiligo frequently affected aspects of their daily lives, including choosing clothes to wear (1956 of 3541 [55.2%]). Most patients (2103 of 3541 [59.4%]) reported concealing their vitiligo frequently. More than half of patients (2078 of 3541 [58.7%]) reported diagnosed mental health conditions, including anxiety (1019 of 3541 [28.8%]) and depression (866 of 3541 [24.5%]). The Patient Health Questionnaire-9 depression screener showed that 55.0% of patients (1948 of 3541) had moderate to severe depressive symptoms; the highest rates were in India (271 of 303 [89.4%]) and among patients with more than 5% affected BSA (1154 of 1602 [72.0%]) and darker skin (987 of 1445 [68.3%]).
Conclusions and relevance: This qualitative study found that, globally, patients with vitiligo reported being substantially affected in their emotional well-being, daily lives, and psychosocial health; the burden was typically greatest among patients with more than 5% affected BSA, darker skin types, and lesions on the face or hands. Survey findings suggest that patients reported having altered their behavior, expressed clear discontent, and have symptoms consistent with depression, which may be underdiagnosed.
Conflict of interest statement
Figures
Similar articles
-
Patient Burden of Nonsegmental Vitiligo: A US Real-World Survey of Dermatologists and Their Patients.Dermatol Ther (Heidelb). 2024 Jun;14(6):1531-1546. doi: 10.1007/s13555-024-01165-5. Epub 2024 May 16. Dermatol Ther (Heidelb). 2024. PMID: 38753072 Free PMC article.
-
Health-Related Quality of Life Burden Among Adults with Vitiligo: Relationship to Disease Severity and Disease Location.Dermatol Ther (Heidelb). 2024 Jun;14(6):1633-1647. doi: 10.1007/s13555-024-01187-z. Epub 2024 Jun 2. Dermatol Ther (Heidelb). 2024. PMID: 38824482 Free PMC article.
-
Vitiligo patient population and disease burden in France: VIOLIN study results from the CONSTANCES cohort.J Eur Acad Dermatol Venereol. 2023 Nov;37(11):2249-2258. doi: 10.1111/jdv.19447. Epub 2023 Aug 31. J Eur Acad Dermatol Venereol. 2023. PMID: 37605309
-
The humanistic burden of vitiligo: a systematic literature review of quality-of-life outcomes.J Eur Acad Dermatol Venereol. 2022 Sep;36(9):1507-1523. doi: 10.1111/jdv.18129. Epub 2022 May 11. J Eur Acad Dermatol Venereol. 2022. PMID: 35366355 Free PMC article. Review.
-
[Quality of life, disease burden and healthcare need of patients with vitiligo].Dermatologie (Heidelb). 2024 May;75(5):404-411. doi: 10.1007/s00105-024-05312-z. Epub 2024 Mar 11. Dermatologie (Heidelb). 2024. PMID: 38466405 Free PMC article. Review. German.
Cited by
-
Patient Burden of Nonsegmental Vitiligo: A US Real-World Survey of Dermatologists and Their Patients.Dermatol Ther (Heidelb). 2024 Jun;14(6):1531-1546. doi: 10.1007/s13555-024-01165-5. Epub 2024 May 16. Dermatol Ther (Heidelb). 2024. PMID: 38753072 Free PMC article.
-
Prevalence of psychiatric comorbidities and treatment initiation in African American pediatric patients with vitiligo: A retrospective, single-center, case-control study.JAAD Int. 2024 Aug 16;17:104-110. doi: 10.1016/j.jdin.2024.07.012. eCollection 2024 Dec. JAAD Int. 2024. PMID: 39399340 Free PMC article.
-
Updates on Potential Therapeutic Approaches for Vitiligo: Janus Kinase Inhibitors and Biologics.J Clin Med. 2023 Dec 4;12(23):7486. doi: 10.3390/jcm12237486. J Clin Med. 2023. PMID: 38068541 Free PMC article. Review.
-
Once-daily upadacitinib versus placebo in adults with extensive non-segmental vitiligo: a phase 2, multicentre, randomised, double-blind, placebo-controlled, dose-ranging study.EClinicalMedicine. 2024 May 31;73:102655. doi: 10.1016/j.eclinm.2024.102655. eCollection 2024 Jul. EClinicalMedicine. 2024. PMID: 38873632 Free PMC article.
-
Error in Supplement 1.JAMA Dermatol. 2024 Jan 1;160(1):118. doi: 10.1001/jamadermatol.2023.4958. JAMA Dermatol. 2024. PMID: 37991742 Free PMC article. No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

