Repurposing of drugs against methyltransferase as potential Zika virus therapies
- PMID: 37188743
- PMCID: PMC10184974
- DOI: 10.1038/s41598-023-33341-6
Repurposing of drugs against methyltransferase as potential Zika virus therapies
Abstract
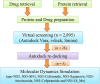
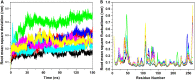
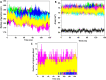
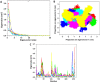
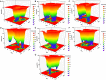
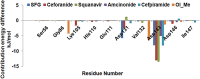
In recent years, the outbreak of infectious disease caused by Zika Virus (ZIKV) has posed a major threat to global public health, calling for the development of therapeutics to treat ZIKV disease. Several possible druggable targets involved in virus replication have been identified. In search of additional potential inhibitors, we screened 2895 FDA-approved compounds using Non-Structural Protein 5 (NS5) as a target utilizing virtual screening of in-silco methods. The top 28 compounds with the threshold of binding energy -7.2 kcal/mol value were selected and were cross-docked on the three-dimensional structure of NS5 using AutoDock Tools. Of the 2895 compounds screened, five compounds (Ceforanide, Squanavir, Amcinonide, Cefpiramide, and Olmesartan_Medoxomil) ranked highest based on filtering of having the least negative interactions with the NS5 and were selected for Molecular Dynamic Simulations (MDS) studies. Various parameters such as RMSD, RMSF, Rg, SASA, PCA and binding free energy were calculated to validate the binding of compounds to the target, ZIKV-NS5. The binding free energy was found to be -114.53, -182.01, -168.19, -91.16, -122.56, and -150.65 kJ mol-1 for NS5-SFG, NS5-Ceforanide, NS5-Squanavir, NS5-Amcinonide, NS5-Cefpiramide, and NS5-Ol_Me complexes respectively. The binding energy calculations suggested Cefpiramide and Olmesartan_Medoxomil (Ol_Me) as the most stable compounds for binding to NS5, indicating a strong rationale for their use as lead compounds for development of ZIKV inhibitors. As these drugs have been evaluated on pharmacokinetics and pharmacodynamics parameters only, in vitro and in vivo testing and their impact on Zika viral cell culture may suggest their clinical trials on ZIKV patients.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Identification of Zika virus NS2B-NS3 protease and NS5 polymerase inhibitors by structure-based virtual screening of FDA-approved drugs.J Biomol Struct Dyn. 2024 Sep;42(15):8073-8088. doi: 10.1080/07391102.2023.2242963. Epub 2023 Aug 1. J Biomol Struct Dyn. 2024. PMID: 37528667
-
Identification and Characterization of Zika Virus NS5 Methyltransferase Inhibitors.Front Cell Infect Microbiol. 2021 Apr 7;11:665379. doi: 10.3389/fcimb.2021.665379. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33898335 Free PMC article.
-
Discovery of Bispecific Lead Compounds from Azadirachta indica against ZIKA NS2B-NS3 Protease and NS5 RNA Dependent RNA Polymerase Using Molecular Simulations.Molecules. 2022 Apr 15;27(8):2562. doi: 10.3390/molecules27082562. Molecules. 2022. PMID: 35458761 Free PMC article.
-
Structure and function of Zika virus NS5 protein: perspectives for drug design.Cell Mol Life Sci. 2018 May;75(10):1723-1736. doi: 10.1007/s00018-018-2751-x. Epub 2018 Feb 8. Cell Mol Life Sci. 2018. PMID: 29423529 Free PMC article. Review.
-
The Potential Role of the ZIKV NS5 Nuclear Spherical-Shell Structures in Cell Type-Specific Host Immune Modulation during ZIKV Infection.Cells. 2019 Nov 26;8(12):1519. doi: 10.3390/cells8121519. Cells. 2019. PMID: 31779251 Free PMC article. Review.
References
-
- Nandy A, Basak SC. The epidemic that shook the world—The Zika virus rampage. Explor. Res. Hypothesis Med. 2017;2:43–56. doi: 10.14218/ERHM.2017.00018. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

