In Vivo Acute Toxicity and Immunomodulation Assessment of a Novel Nutraceutical in Mice
- PMID: 37111777
- PMCID: PMC10144505
- DOI: 10.3390/pharmaceutics15041292
In Vivo Acute Toxicity and Immunomodulation Assessment of a Novel Nutraceutical in Mice
Abstract
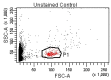
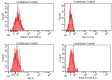
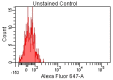
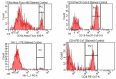
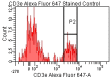
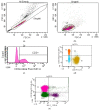
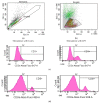
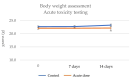
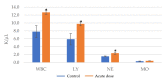
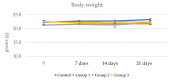
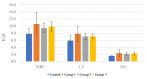
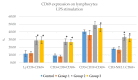
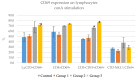
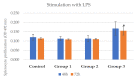
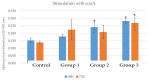
Achieving and maintaining a well-balanced immune system has righteously become an insightful task for the general population and an even more fundamental goal for those affected by immune-related diseases. Since our immune functions are indispensable in defending the body against pathogens, diseases and other external attacks, while playing a vital role in maintaining health and modulating the immune response, we require an on-point grasp of their shortcoming as a foundation for the development of functional foods and novel nutraceuticals. Seeing that immunoceuticals are considered effective in improving immune functions and reducing the incidence of immunological disorders, the main focus of this study was to assess the immunomodulatory properties and possible acute toxicity of a novel nutraceutical with active substances of natural origin on C57BL/6 mice for 21 days. We evaluated the potential hazards (microbial contamination and heavy metals) of the novel nutraceutical and addressed the acute toxicity according to OECD guidelines of a 2000 mg/kg dose on mice for 21 days. The immunomodulatory effect was assessed at three concentrations (50 mg/kg, 100 mg/kg and 200 mg/kg) by determining body and organ indexes through a leukocyte analysis; flow cytometry immunophenotyping of lymphocytes populations and their subpopulations (T lymphocytes (LyCD3+), cytotoxic suppressor T lymphocytes (CD3+CD8+), helper T lymphocytes (CD3+CD4+), B lymphocytes (CD3-CD19+) and NK cells (CD3-NK1.1.+); and the expression of the CD69 activation marker. The results obtained for the novel nutraceutical referred to as ImunoBoost indicated no acute toxicity, an increased number of lymphocytes and the stimulation of lymphocyte activation and proliferation, demonstrating its immunomodulatory effect. The safe human consumption dose was established at 30 mg/day.
Keywords: Echinacea purpurea; Vaccinium myrtillus; acute toxicity; animal studies; hydrolyzed collagen; immunomodulation; nutraceuticals; royal jelly.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
[Lymphocyte activation markers in patients with ovarian cancer].Ginekol Pol. 2012 Oct;83(10):737-43. Ginekol Pol. 2012. PMID: 23383558 Polish.
-
Effect of blood transfusion during radiotherapy on the immune function of patients with cancer of the uterine cervix: role of interleukin-10.Int J Radiat Oncol Biol Phys. 2002 Dec 1;54(5):1345-55. doi: 10.1016/s0360-3016(02)03757-4. Int J Radiat Oncol Biol Phys. 2002. PMID: 12459356
-
Hypergravity-induced immunomodulation in a rodent model: lymphocytes and lymphoid organs.J Gravit Physiol. 2002 Dec;9(2):15-27. J Gravit Physiol. 2002. PMID: 14638456
-
[Deep lung--cellular reaction to HIV].Rev Port Pneumol. 2007 Mar-Apr;13(2):175-212. Rev Port Pneumol. 2007. PMID: 17492233 Review. Portuguese.
-
Safety and nutritional assessment of GM plants and derived food and feed: the role of animal feeding trials.Food Chem Toxicol. 2008 Mar;46 Suppl 1:S2-70. doi: 10.1016/j.fct.2008.02.008. Epub 2008 Feb 13. Food Chem Toxicol. 2008. PMID: 18328408 Review.
Cited by
-
Immunomodulatory Effect of Phytoactive Compounds on Human Health: A Narrative Review Integrated with Bioinformatics Approach.Curr Top Med Chem. 2024;24(12):1075-1100. doi: 10.2174/0115680266274272240321065039. Curr Top Med Chem. 2024. PMID: 38551050 Review.
References
-
- Brandelli A., Daroit D.J., Corrêa A.P.F. Whey as a source of peptides with remarkable biological activities. Food Res. Int. 2015;73:149–161. doi: 10.1016/j.foodres.2015.01.016. - DOI
-
- Reyes-Díaz A., González-Córdova A.F., Hernández-Mendoza A., Reyes-Díaz R., Vallejo-Cordoba B. Immunomodulation by hydrolysates and peptides derived from milk proteins. Int. J. Dairy Technol. 2018;71:1–9. doi: 10.1111/1471-0307.12421. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

