Loss of SQSTM1/p62 Induces Obesity and Exacerbates Alcohol-Induced Liver Injury in Aged Mice
- PMID: 36754207
- PMCID: PMC10036741
- DOI: 10.1016/j.jcmgh.2023.01.016
Loss of SQSTM1/p62 Induces Obesity and Exacerbates Alcohol-Induced Liver Injury in Aged Mice
Abstract
Background: Alcohol-associated liver disease (ALD) is a worldwide health problem, of which the effective treatment is still lacking. Both detrimental and protective roles of adipose tissue have been implicated in ALD. Although alcohol increases adipose tissue lipolysis to promote alcohol-induced liver injury, alcohol also activates brown adipose tissue (BAT) thermogenesis as an adaptive response in protecting against alcohol-induced liver injury. Moreover, aging and obesity are also risk factors for ALD. In the present study, we investigated the effects of autophagy receptor protein SQSTM1/p62 on adipose tissue and obesity in alcohol-induced liver injury in both young and aged mice.
Methods: Young and aged whole-body SQSTM1/p62 knockout (KO) and their age-matched wild-type (WT) mice were subjected to chronic plus binge (Gao-binge) alcohol feeding. Blood, adipose and liver tissues were collected for biochemical and histologic analysis.
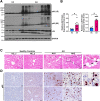
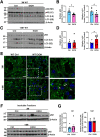
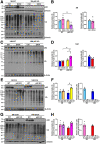
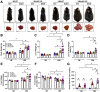
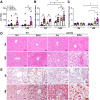
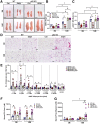
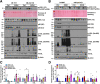
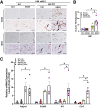
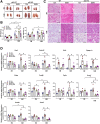
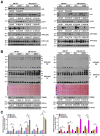
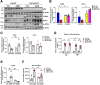
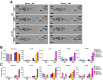
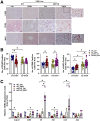
Results: Aged but not young SQSTM1/p62 KO mice had significantly increased body weight and fat mass compared with the matched WT mice. Gao-binge alcohol feeding induced white adipose atrophy and decreased levels of SQSTM1/p62 levels in adipose tissue in aged WT mice. SQSTM1/p62 KO aged mice were resistant to Gao-binge alcohol-induced white adipose atrophy. Alcohol feeding increased the expression of thermogenic genes in WT mouse BAT, which was significantly blunted in SQSTM1/p62 KO aged mice. Alcohol-fed aged SQSTM1/p62 KO mice showed significantly higher levels of serum alanine aminotransferase, hepatic triglyceride, and inflammation compared with young and aged WT mice fed with alcohol. Alcohol-fed SQSTM1/p62 KO mice also increased secretion of proinflammatory and angiogenic adipokines that may promote alcohol-induced liver injury.
Conclusions: Loss of SQSTM1/p62 in aged mice leads to obesity and impairs alcohol-induced BAT adaptation, resulting in exacerbated alcohol-induced liver injury in mice.
Keywords: ALD; Adipokine; Adipose Tissue; Autophagy; Steatosis.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Loss of SQSTM1/p62 Induces Obesity and Exacerbates Alcohol-induced Liver Injury in Aged Mice.FASEB J. 2022 May;36 Suppl 1. doi: 10.1096/fasebj.2022.36.S1.R2198. FASEB J. 2022. PMID: 35723871
-
Role of Mechanistic Target of Rapamycin and Autophagy in Alcohol-Induced Adipose Atrophy and Liver Injury.Am J Pathol. 2020 Jan;190(1):158-175. doi: 10.1016/j.ajpath.2019.09.023. Epub 2019 Nov 13. Am J Pathol. 2020. PMID: 31733185 Free PMC article.
-
Liver-adipose tissue crosstalk in alcohol-associated liver disease: The role of mTOR.Liver Res. 2022 Dec;6(4):227-237. doi: 10.1016/j.livres.2022.11.006. Epub 2022 Nov 18. Liver Res. 2022. PMID: 37124481 Free PMC article.
-
n-3 Polyunsaturated fatty acids for the management of alcoholic liver disease: A critical review.Crit Rev Food Sci Nutr. 2019;59(sup1):S116-S129. doi: 10.1080/10408398.2018.1544542. Epub 2018 Dec 22. Crit Rev Food Sci Nutr. 2019. PMID: 30580553 Review.
-
Alcohol, adipose tissue and liver disease: mechanistic links and clinical considerations.Nat Rev Gastroenterol Hepatol. 2018 Jan;15(1):50-59. doi: 10.1038/nrgastro.2017.116. Epub 2017 Sep 20. Nat Rev Gastroenterol Hepatol. 2018. PMID: 28930290 Review.
Cited by
-
Recent insights into the pathogenesis and therapeutic targets of chronic liver diseases.eGastroenterology. 2023 Oct;1(2):e100020. doi: 10.1136/egastro-2023-100020. eGastroenterology. 2023. PMID: 38074919 Free PMC article.
-
Alcohol-associated liver disease.J Clin Invest. 2024 Feb 1;134(3):e176345. doi: 10.1172/JCI176345. J Clin Invest. 2024. PMID: 38299591 Free PMC article. Review.
-
SQSTM1/p62 and Hepatic Mallory-Denk Body Formation in Alcohol-Associated Liver Disease.Am J Pathol. 2023 Oct;193(10):1415-1426. doi: 10.1016/j.ajpath.2023.02.015. Epub 2023 Mar 9. Am J Pathol. 2023. PMID: 36906265 Free PMC article. Review.
-
High-throughput screening of novel TFEB agonists in protecting against acetaminophen-induced liver injury in mice.Acta Pharm Sin B. 2024 Jan;14(1):190-206. doi: 10.1016/j.apsb.2023.10.017. Epub 2023 Oct 29. Acta Pharm Sin B. 2024. PMID: 38261809 Free PMC article.
-
Protective Effects of Lycium ruthenicum Murray against Acute Alcoholic Liver Disease in Mice via the Nrf2/HO-1/NF-κB Signaling Pathway.Pharmaceuticals (Basel). 2024 Apr 13;17(4):497. doi: 10.3390/ph17040497. Pharmaceuticals (Basel). 2024. PMID: 38675458 Free PMC article.
References
-
- Paik J.M., Golabi P., Younossi Y., et al. Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of NAFLD. Hepatology. 2020;72:1605–1616. - PubMed
-
- Mokdad A.H., Marks J.S., Stroup D.F., et al. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

