Nobiletin suppresses cholangiocarcinoma proliferation via inhibiting GSK3β
- PMID: 36263164
- PMCID: PMC9576508
- DOI: 10.7150/ijbs.78345
Nobiletin suppresses cholangiocarcinoma proliferation via inhibiting GSK3β
Abstract
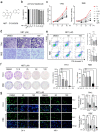
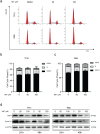
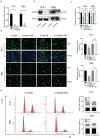
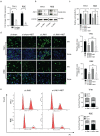
Background: Cholangiocarcinoma (CCA) is a type of hepatobiliary cancer characterized by uncontrolled cell proliferation, with a poor prognosis and high mortality. Nobiletin (NBT) is a promising anti-tumor compound derived from the peels of oranges and other citrus plants, citrus plant. But the effect of NBT on CCA remains unknown. Results: Our data showed that NBT suppressed CCA cell proliferation in vitro and in vivo. Colony formation and Edu assay indicated that NBT inhibited cell proliferation. Cell cycle analysis showed that NBT arrested the cell cycle in G0/G1 phase. Target prediction showed that GSK3β was a direct target. Western blot and immunofluorescence confirmed that NBT reduced the phosphorylation of GSK3β. The antiproliferative effect of NBT was intercepted in GSK3β knockdown CCA cells. The cellular thermal shift assay (CETSA) showed NBT directly bound to GSK3β. Finally, NBT showed an anti-proliferative effect in tumor-bearing mice with no hepatotoxicity. Conclusion: NBT could inhibit CCA proliferation, and the pharmacological activity of NBT in CCA was attributed to its direct binding to GSK3β. We suggested that NBT might be a potential natural medicine in CCA treatment.
Keywords: Cholangiocarcinoma; GSK3β; Nobiletin; natural medicine; β-catenin.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

Similar articles
-
β-escin reverses multidrug resistance through inhibition of the GSK3β/β-catenin pathway in cholangiocarcinoma.World J Gastroenterol. 2015 Jan 28;21(4):1148-57. doi: 10.3748/wjg.v21.i4.1148. World J Gastroenterol. 2015. PMID: 25632187 Free PMC article.
-
Wnt/β-catenin signaling as an emerging potential key pharmacological target in cholangiocarcinoma.Biosci Rep. 2020 Mar 27;40(3):BSR20193353. doi: 10.1042/BSR20193353. Biosci Rep. 2020. PMID: 32140709 Free PMC article. Review.
-
Knockdown of tripartite motif 59 (TRIM59) inhibits proliferation in cholangiocarcinoma via the PI3K/AKT/mTOR signalling pathway.Gene. 2019 May 25;698:50-60. doi: 10.1016/j.gene.2019.02.044. Epub 2019 Feb 27. Gene. 2019. PMID: 30822475
-
Cannabidiol and Cannabigerol Inhibit Cholangiocarcinoma Growth In Vitro via Divergent Cell Death Pathways.Biomolecules. 2022 Jun 20;12(6):854. doi: 10.3390/biom12060854. Biomolecules. 2022. PMID: 35740979 Free PMC article.
-
Combined targeting of AKT and mTOR using MK-2206 and RAD001 is synergistic in the treatment of cholangiocarcinoma.Int J Cancer. 2013 Nov;133(9):2065-76. doi: 10.1002/ijc.28214. Epub 2013 May 29. Int J Cancer. 2013. PMID: 23588885
Cited by
-
Digitoxin inhibits ICC cell properties via the NF‑κB/ST6GAL1 signaling pathway.Oncol Rep. 2024 Aug;52(2):103. doi: 10.3892/or.2024.8762. Epub 2024 Jun 28. Oncol Rep. 2024. PMID: 38940341 Free PMC article.
-
METTL protein family: focusing on the occurrence, progression and treatment of cancer.Biomark Res. 2024 Sep 17;12(1):105. doi: 10.1186/s40364-024-00652-3. Biomark Res. 2024. PMID: 39289775 Free PMC article. Review.
-
Design, synthesis and biological evaluation of a novel colchicine-magnolol hybrid for inhibiting the growth of Lewis lung carcinoma in Vitro and in Vivo.Front Chem. 2022 Dec 13;10:1094019. doi: 10.3389/fchem.2022.1094019. eCollection 2022. Front Chem. 2022. PMID: 36583151 Free PMC article.
-
γ-aminobutyric acid B2 receptor: A potential therapeutic target for cholangiocarcinoma in patients with diabetes mellitus.World J Gastroenterol. 2023 Jul 28;29(28):4416-4432. doi: 10.3748/wjg.v29.i28.4416. World J Gastroenterol. 2023. PMID: 37576707 Free PMC article.
-
UBA3 promotes the occurrence and metastasis of intrahepatic cholangiocarcinoma through MAPK signaling pathway.Acta Biochim Biophys Sin (Shanghai). 2024 Feb 25;56(2):199-209. doi: 10.3724/abbs.2024014. Acta Biochim Biophys Sin (Shanghai). 2024. PMID: 38298057 Free PMC article.
References
-
- Khan SA, Davidson BR, Goldin RD, Heaton N, Karani J, Pereira SP. et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61(12):1657–69. - PubMed
-
- Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P. et al. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA) Nat Rev Gastroenterol Hepatol. 2016;13(5):261–80. - PubMed
-
- Khan SA, Emadossadaty S, Ladep NG, Thomas HC, Elliott P, Taylor-Robinson SD. et al. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol. 2012;56(4):848–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

