Pathophysiology of Immune Checkpoint Inhibitor-Induced Myocarditis
- PMID: 36139654
- PMCID: PMC9497311
- DOI: 10.3390/cancers14184494
Pathophysiology of Immune Checkpoint Inhibitor-Induced Myocarditis
Abstract
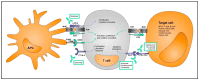
Immune checkpoint inhibitors (ICIs) have recently emerged as strong therapies for a broad spectrum of cancers being the first-line treatment for many of them, even improving the prognosis of malignancies that were considered untreatable. This therapy is based on the administration of monoclonal antibodies targeting inhibitory T-cell receptors, which boost the immune system and prevent immune evasion. However, non-specific T-cell de-repression can result in a wide variety of immune-related adverse events (irAEs), including gastrointestinal, endocrine, and dermatologic, with a smaller proportion of these having the potential for fatal outcomes such as neurotoxicity, pulmonary toxicity, and cardiotoxicity. In recent years, alarm has been raised about cardiotoxicity as it has the highest mortality rate when myocarditis develops. However, due to the difficulty in diagnosing this cardiac condition and the lack of clinical guidelines for the management of cardiovascular disease in patients on therapy with ICIs, early detection of myocarditis has become a challenge in these patients. In this review we outline the mechanisms of tolerance by which this fatal cardiomyopathy may develop in selected cancer patients treated with ICIs, summarize preclinical models of the disease that will allow the development of more accurate strategies for its detection and treatment, and discuss the challenges in the future to decrease the risks of its development with better decision making in susceptible patients.
Keywords: ICI-myocarditis; T cells; anti-CTLA-4; anti-PD-1; cancer therapy; cardiotoxicity; immunotherapy; irAEs; myocarditis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
A Systematic Review of the Mechanisms Involved in Immune Checkpoint Inhibitors Cardiotoxicity and Challenges to Improve Clinical Safety.Front Cell Dev Biol. 2022 Mar 30;10:851032. doi: 10.3389/fcell.2022.851032. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35433707 Free PMC article.
-
Cardiotoxicity associated with immune checkpoint inhibitor therapy: a meta-analysis.Eur J Heart Fail. 2021 Oct;23(10):1739-1747. doi: 10.1002/ejhf.2289. Epub 2021 Jul 29. Eur J Heart Fail. 2021. PMID: 34196077
-
Immune checkpoint inhibitors associated cardiovascular immune-related adverse events.Front Immunol. 2024 Feb 5;15:1340373. doi: 10.3389/fimmu.2024.1340373. eCollection 2024. Front Immunol. 2024. PMID: 38375475 Free PMC article. Review.
-
Clinical Characteristics and Treatment of Immune-Related Adverse Events of Immune Checkpoint Inhibitors.Immune Netw. 2020 Feb 17;20(1):e9. doi: 10.4110/in.2020.20.e9. eCollection 2020 Feb. Immune Netw. 2020. PMID: 32158597 Free PMC article. Review.
-
Cardiotoxicity of Immune Checkpoint Inhibitors.Curr Oncol Rep. 2021 May 3;23(7):79. doi: 10.1007/s11912-021-01070-6. Curr Oncol Rep. 2021. PMID: 33937956 Free PMC article. Review.
Cited by
-
Multimodality imaging in cardio-oncology: the added value of CMR and CCTA.Br J Radiol. 2023 Oct;96(1150):20220999. doi: 10.1259/bjr.20220999. Epub 2023 Jul 26. Br J Radiol. 2023. PMID: 37493228 Free PMC article. Review.
-
Complete and early response to cemiplimab associated to severe immune toxicity in advanced cervical cancer: a case report.Front Immunol. 2023 Dec 13;14:1303893. doi: 10.3389/fimmu.2023.1303893. eCollection 2023. Front Immunol. 2023. PMID: 38193091 Free PMC article.
-
Successful treatment with carboplatin and paclitaxel in melanoma progression after immune-related adverse events.Immunotherapy. 2023 Sep;15(13):993-999. doi: 10.2217/imt-2022-0213. Epub 2023 Jul 31. Immunotherapy. 2023. PMID: 37525573 Free PMC article.
-
Drug Selection and Posology, Optimal Therapies and Risk/Benefit Assessment in Medicine: The Paradigm of Iron-Chelating Drugs.Int J Mol Sci. 2023 Nov 25;24(23):16749. doi: 10.3390/ijms242316749. Int J Mol Sci. 2023. PMID: 38069073 Free PMC article. Review.
-
Reconstitution of peripheral blood T cell receptor β immune repertoire in immune checkpoint inhibitors associated myocarditis.Cardiooncology. 2024 Jun 11;10(1):35. doi: 10.1186/s40959-024-00230-4. Cardiooncology. 2024. PMID: 38863010 Free PMC article.
References
-
- Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. - DOI - PubMed
-
- Rubio-Infante N., Ramírez-Flores Y.A., Castillo E.C., Lozano O., García-Rivas G., Torre-Amione G. A Systematic Review of the Mechanisms Involved in Immune Checkpoint Inhibitors Cardiotoxicity and Challenges to Improve Clinical Safety. Front. Cell Dev. Biol. 2022;10:851032. doi: 10.3389/fcell.2022.851032. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

