IL-17 and IL-17-producing cells in protection versus pathology
- PMID: 35790881
- PMCID: PMC9255545
- DOI: 10.1038/s41577-022-00746-9
IL-17 and IL-17-producing cells in protection versus pathology
Abstract
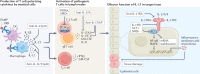
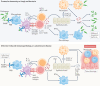
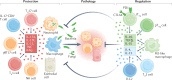
IL-17 cytokine family members have diverse biological functions, promoting protective immunity against many pathogens but also driving inflammatory pathology during infection and autoimmunity. IL-17A and IL-17F are produced by CD4+ and CD8+ T cells, γδ T cells, and various innate immune cell populations in response to IL-1β and IL-23, and they mediate protective immunity against fungi and bacteria by promoting neutrophil recruitment, antimicrobial peptide production and enhanced barrier function. IL-17-driven inflammation is normally controlled by regulatory T cells and the anti-inflammatory cytokines IL-10, TGFβ and IL-35. However, if dysregulated, IL-17 responses can promote immunopathology in the context of infection or autoimmunity. Moreover, IL-17 has been implicated in the pathogenesis of many other disorders with an inflammatory basis, including cardiovascular and neurological diseases. Consequently, the IL-17 pathway is now a key drug target in many autoimmune and chronic inflammatory disorders; therapeutic monoclonal antibodies targeting IL-17A, both IL-17A and IL-17F, the IL-17 receptor, or IL-23 are highly effective in some of these diseases. However, new approaches are needed to specifically regulate IL-17-mediated immunopathology in chronic inflammation and autoimmunity without compromising protective immunity to infection.
© 2022. Springer Nature Limited.
Conflict of interest statement
K.H.G.M. is a co-founder and shareholder in a Biotech start-up company involved in the development of anti-inflammatory therapeutics.
Figures
Similar articles
-
Interleukin-17 family cytokines in protective immunity against infections: role of hematopoietic cell-derived and non-hematopoietic cell-derived interleukin-17s.Microbiol Immunol. 2018 Jan;62(1):1-13. doi: 10.1111/1348-0421.12560. Microbiol Immunol. 2018. PMID: 29205464 Review.
-
The paradigm of IL-23-independent production of IL-17F and IL-17A and their role in chronic inflammatory diseases.Front Immunol. 2023 Aug 4;14:1191782. doi: 10.3389/fimmu.2023.1191782. eCollection 2023. Front Immunol. 2023. PMID: 37600764 Free PMC article. Review.
-
Selective targeting of PI3Kδ suppresses human IL-17-producing T cells and innate-like lymphocytes and may be therapeutic for IL-17-mediated diseases.J Autoimmun. 2020 Jul;111:102435. doi: 10.1016/j.jaut.2020.102435. Epub 2020 Apr 29. J Autoimmun. 2020. PMID: 32360069
-
IL-17C/IL-17 Receptor E Signaling in CD4+ T Cells Promotes TH17 Cell-Driven Glomerular Inflammation.J Am Soc Nephrol. 2018 Apr;29(4):1210-1222. doi: 10.1681/ASN.2017090949. Epub 2018 Feb 26. J Am Soc Nephrol. 2018. PMID: 29483158 Free PMC article.
-
Interleukin-17A Serves a Priming Role in Autoimmunity by Recruiting IL-1β-Producing Myeloid Cells that Promote Pathogenic T Cells.Immunity. 2020 Feb 18;52(2):342-356.e6. doi: 10.1016/j.immuni.2020.01.002. Epub 2020 Feb 4. Immunity. 2020. PMID: 32023490
Cited by
-
Gut-first Parkinson's disease is encoded by gut dysbiome.Mol Neurodegener. 2024 Oct 24;19(1):78. doi: 10.1186/s13024-024-00766-0. Mol Neurodegener. 2024. PMID: 39449004 Free PMC article.
-
Maternal consumption of a high-fat diet modulates the inflammatory response in their offspring, mediated by the M1 muscarinic receptor.Front Immunol. 2023 Dec 18;14:1273556. doi: 10.3389/fimmu.2023.1273556. eCollection 2023. Front Immunol. 2023. PMID: 38193079 Free PMC article.
-
Advances in the study of IL-17 in neurological diseases and mental disorders.Front Neurol. 2023 Nov 16;14:1284304. doi: 10.3389/fneur.2023.1284304. eCollection 2023. Front Neurol. 2023. PMID: 38046578 Free PMC article. Review.
-
Molecular interactions of adaptor protein PSTPIP2 control neutrophil-mediated responses leading to autoinflammation.Front Immunol. 2022 Dec 20;13:1035226. doi: 10.3389/fimmu.2022.1035226. eCollection 2022. Front Immunol. 2022. PMID: 36605205 Free PMC article.
-
IL-17: Balancing Protective Immunity and Pathogenesis.J Immunol Res. 2023 Aug 12;2023:3360310. doi: 10.1155/2023/3360310. eCollection 2023. J Immunol Res. 2023. PMID: 37600066 Free PMC article. Review.
References
-
- Rouvier E, Luciani MF, Mattéi MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J. Immunol. 1993;150:5445–5456. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

