Melatonin and andrographolide synergize to inhibit the colospheroid phenotype by targeting Wnt/beta-catenin signaling
- PMID: 35619550
- PMCID: PMC9288490
- DOI: 10.1111/jpi.12808
Melatonin and andrographolide synergize to inhibit the colospheroid phenotype by targeting Wnt/beta-catenin signaling
Abstract
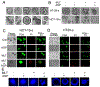
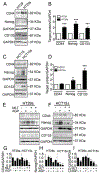
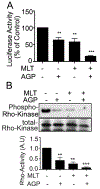
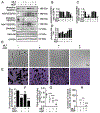
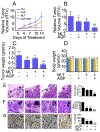
β-catenin signaling, and angiogenesis are associated with colospheroid (CSC), development. CSCs, spheroids derived from colon cancer cells, are responsible for metastasis, drug resistance, and disease recurrence. Whether dysregulating β-catenin and inhibiting angiogenesis reduce CSC growth is unknown. In this study, the molecular mechanism of CSC growth inhibition was evaluated using a novel combination of melatonin (MLT) and andrographolide (AGP). These drugs have anticarcinogenic, antioxidant, and antimetastatic properties. CSCs were obtained from two metastatic colon cancer cell lines (HT29 and HCT-15). The viability and stemness were monitored (FDA propidium iodide staining and immunoblot for CD44, CD133, Nanog, Sox2, and Oct4). The drug combination synergistically diminished stemness via increased reactive oxygen species (ROS) levels, reduced mitochondrial membrane potential and ATP level. MLT + AGP induced cell death by inhibiting β-catenin expression and its downregulatory signals, Cyclin D1, c-Myc. MLT + AGP treated cells exhibited translocation of phospho-β-catenin to the nucleus and dephosphorylated-β-catenin. Downregulation of β-catenin activation and its transcription factors (TCF4 and LEF1) and GTP binding/G-protein related activity were found in the dual therapy. Angiogenic inhibition is consistent with downregulation of VEGF messenger RNA transcripts (VEGF189), phosphorylated VEGF receptor protein expression, matrigel invasion, and capillary tube inhibition. In vivo, the intravenous injection of MLT + AGP slowed HT29 metastatic colon cancer. Histopathology indicated significant reduction in microvascular density and tumor index. Immunohistochemistry for caspase 7, and β-catenin found increased apoptosis and downregulation of β-catenin signals. The mechanism(s) of decreased colospheroids growth were the inhibition of the Wnt/β-catenin pathway. Our results provide a rationale for using MLT in combination with AGP for the inhibition of CRCs.
Keywords: Wnt/β-catenin signals; andrographolide; angiogenesis; colospheroids; melatonin; xenograft.
© 2022 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures


Similar articles
-
Synergistic potential of dual andrographolide and melatonin targeting of metastatic colon cancer cells: Using the Chou-Talalay combination index method.Eur J Pharmacol. 2021 Apr 15;897:173919. doi: 10.1016/j.ejphar.2021.173919. Epub 2021 Feb 9. Eur J Pharmacol. 2021. PMID: 33577837
-
MicroRNA-1 down-regulates proliferation and migration of breast cancer stem cells by inhibiting the Wnt/β-catenin pathway.Oncotarget. 2015 Dec 8;6(39):41638-49. doi: 10.18632/oncotarget.5873. Oncotarget. 2015. PMID: 26497855 Free PMC article.
-
The NOP14 nucleolar protein suppresses the function and stemness of melanoma stem-like cells through Wnt/beta-catenin signaling inactivation.Bioengineered. 2022 Mar;13(3):7648-7658. doi: 10.1080/21655979.2022.2050491. Bioengineered. 2022. PMID: 35282769 Free PMC article.
-
Radiation-induced glucocorticoid receptor promotes CD44+ prostate cancer stem cell growth through activation of SGK1-Wnt/β-catenin signaling.J Mol Med (Berl). 2019 Aug;97(8):1169-1182. doi: 10.1007/s00109-019-01807-8. Epub 2019 Jun 11. J Mol Med (Berl). 2019. Retraction in: J Mol Med (Berl). 2022 Jan;100(1):149. doi: 10.1007/s00109-021-02173-0 PMID: 31187175 Retracted.
-
Melatonin as a Promising Agent for Cancer Treatment: Insights into its Effects on the Wnt/beta-catenin Signaling Pathway.Curr Med Chem. 2024;31(11):1315-1331. doi: 10.2174/0929867330666230409141957. Curr Med Chem. 2024. PMID: 37031385 Review.
Cited by
-
A sponge-like nanofiber melatonin-loaded scaffold accelerates vascularized bone regeneration via improving mitochondrial energy metabolism.Mater Today Bio. 2024 May 3;26:101078. doi: 10.1016/j.mtbio.2024.101078. eCollection 2024 Jun. Mater Today Bio. 2024. PMID: 38765244 Free PMC article.
-
GPX2 acts as an oncogene and cudraflavone C has an anti-tumor effect by suppressing GPX2-dependent Wnt/β-catenin pathway in colorectal cancer cells.Naunyn Schmiedebergs Arch Pharmacol. 2024 Feb;397(2):1115-1125. doi: 10.1007/s00210-023-02668-2. Epub 2023 Aug 23. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 37610461
-
Melatonin and Its Role in the Epithelial-to-Mesenchymal Transition (EMT) in Cancer.Cancers (Basel). 2024 Feb 27;16(5):956. doi: 10.3390/cancers16050956. Cancers (Basel). 2024. PMID: 38473317 Free PMC article. Review.
-
Restoration of follicular β-catenin signaling by mesenchymal stem cells promotes hair growth in mice with androgenetic alopecia.Stem Cell Res Ther. 2024 Nov 19;15(1):439. doi: 10.1186/s13287-024-04051-1. Stem Cell Res Ther. 2024. PMID: 39563459 Free PMC article.
-
Hypothermia increases cold-inducible protein expression and improves cerebellar-dependent learning after hypoxia ischemia in the neonatal rat.Pediatr Res. 2023 Aug;94(2):539-546. doi: 10.1038/s41390-023-02535-z. Epub 2023 Feb 21. Pediatr Res. 2023. PMID: 36810641 Free PMC article.
References
-
- Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23(10):1124–1134. - PubMed
-
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. - PubMed
-
- Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–648. - PubMed
-
- Bjerkvig R, Tysnes BB, Aboody KS, Najbauer J, Terzis AJ. Opinion: the origin of the cancer stem cell: current controversies and new insights. Nat Rev Cancer. 2005;5(11):899–904. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

