Nobiletin, a citrus polymethoxyflavone, enhances the effects of bicalutamide on prostate cancer cells via down regulation of NF-κB, STAT3, and ERK activation
- PMID: 35498570
- PMCID: PMC9050343
- DOI: 10.1039/c9ra10020b
Nobiletin, a citrus polymethoxyflavone, enhances the effects of bicalutamide on prostate cancer cells via down regulation of NF-κB, STAT3, and ERK activation
Abstract
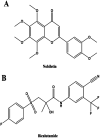
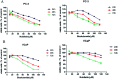
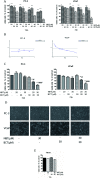
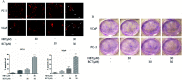
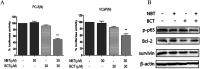
Natural products have shown potential to be combined with current cancer therapies to improve patient outcomes. Nobiletin (NBT) is a citrus polymethoxyflavone and has been shown to exert an anticancer effect in various cancer cells. We investigated the effects and mechanisms of NBT in combination with bicalutamide (BCT), a commonly used anti-androgen drug in prostate cancer therapy, on prostate cancer cells. Our results demonstrate that the combined treatment with NBT and BCT produces an enhanced inhibitory effect on the growth of prostate cancer cells compared to either compound alone. The synergistic action of NBT and BCT was confirmed using isobologram analysis. Moreover, this study has shown that NBT and BCT synergistically inhibited colony formation and migration as well as induced apoptosis. Mechanistic studies demonstrate that NBT and BCT combination reduced key cellular signaling regulators including: p-Erk/Erk, p-STAT3/STAT3 and NF-κB. Overall, these results suggest that NBT combination with BCT may be an effective treatment for prostate cancer.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Synergistic chemopreventive effects of nobiletin and atorvastatin on colon carcinogenesis.Carcinogenesis. 2017 Apr 1;38(4):455-464. doi: 10.1093/carcin/bgx018. Carcinogenesis. 2017. PMID: 28207072 Free PMC article.
-
A metabolite of nobiletin, 4'-demethylnobiletin and atorvastatin synergistically inhibits human colon cancer cell growth by inducing G0/G1 cell cycle arrest and apoptosis.Food Funct. 2018 Jan 24;9(1):87-95. doi: 10.1039/c7fo01155e. Food Funct. 2018. PMID: 29063088 Free PMC article.
-
Nobiletin Enhances Chemosensitivity to Adriamycin through Modulation of the Akt/GSK3β/β⁻Catenin/MYCN/MRP1 Signaling Pathway in A549 Human Non-Small-Cell Lung Cancer Cells.Nutrients. 2018 Nov 26;10(12):1829. doi: 10.3390/nu10121829. Nutrients. 2018. PMID: 30486290 Free PMC article.
-
Nobiletin Inhibits CD36-Dependent Tumor Angiogenesis, Migration, Invasion, and Sphere Formation Through the Cd36/Stat3/Nf-Κb Signaling Axis.Nutrients. 2018 Jun 15;10(6):772. doi: 10.3390/nu10060772. Nutrients. 2018. PMID: 29914089 Free PMC article.
-
Combination of curcumin and bicalutamide enhanced the growth inhibition of androgen-independent prostate cancer cells through SAPK/JNK and MEK/ERK1/2-mediated targeting NF-κB/p65 and MUC1-C.J Exp Clin Cancer Res. 2015 May 15;34(1):46. doi: 10.1186/s13046-015-0168-z. J Exp Clin Cancer Res. 2015. PMID: 25971429 Free PMC article.
Cited by
-
STAT3 and Its Pathways' Dysregulation-Underestimated Role in Urological Tumors.Cells. 2022 Sep 27;11(19):3024. doi: 10.3390/cells11193024. Cells. 2022. PMID: 36230984 Free PMC article. Review.
-
Recent advances in flavonoid compounds for the treatment of prostate cancer.Mol Biol Rep. 2024 May 11;51(1):653. doi: 10.1007/s11033-024-09567-6. Mol Biol Rep. 2024. PMID: 38734766 Review.
-
Hesperidin Suppresses the Proliferation of Prostate Cancer Cells by Inducing Oxidative Stress and Disrupting Ca2+ Homeostasis.Antioxidants (Basel). 2022 Aug 23;11(9):1633. doi: 10.3390/antiox11091633. Antioxidants (Basel). 2022. PMID: 36139707 Free PMC article.
-
ROR activation by Nobiletin enhances antitumor efficacy via suppression of IκB/NF-κB signaling in triple-negative breast cancer.Cell Death Dis. 2022 Apr 19;13(4):374. doi: 10.1038/s41419-022-04826-5. Cell Death Dis. 2022. PMID: 35440077 Free PMC article.
-
Nobiletin as an inducer of programmed cell death in cancer: a review.Apoptosis. 2022 Jun;27(5-6):297-310. doi: 10.1007/s10495-022-01721-4. Epub 2022 Mar 21. Apoptosis. 2022. PMID: 35312885 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

