Beneficial effects of a nobiletin-rich formulated supplement of Sikwasa (C. depressa) peel on cognitive function in elderly Japanese subjects; A multicenter, randomized, double-blind, placebo-controlled study
- PMID: 34925812
- PMCID: PMC8645756
- DOI: 10.1002/fsn3.2640
Beneficial effects of a nobiletin-rich formulated supplement of Sikwasa (C. depressa) peel on cognitive function in elderly Japanese subjects; A multicenter, randomized, double-blind, placebo-controlled study
Abstract
Background: Nobiletin exerts beneficial effects on cognitive function in various animal models of Alzheimer's disease. The present study aimed to investigate the benefits and safety of a combination food of nobiletin-rich extract from C. depressa peel for healthy elderly subjects.
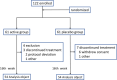
Methods: The nobiletin-containing test food (Nobilex®) comprised high-purity nobiletin powder combined with dried root powder of K. parviflora and dried lead powder of P. japonicum and was administered to elderly Japanese subjects once a day for 16 weeks. The Japanese version of the Wechsler Memory Scaled-Revised (WMS-R) was used as a primary evaluation item for the assessment of global memory. Data from a protocol-matched population (Per Protocol Set: PPS) (n = 108) were analyzed.
Results: The scores of "general memory" or "visual memory" in the indices of WMS-R were significantly higher in the nobiletin-containing test food group than in the placebo group. The difference in the total WMS-R score was significantly higher in the test-food group (9.0 ± 7.20) than in the placebo group (5.9 ± 7.70). An age-stratified analysis of the WMS-R test showed similar changes in subjects aged ≦74 years to those in the overall subject population. In the stratified analysis involving subjects with an MMSE-J score of between 24 and 28, the "figural memory" subscale score in WMS-R was significantly higher in the test food group than in the placebo group.
Conclusion: The present results indicate that the nobiletin-containing test food is beneficial for improving memory dysfunction in healthy elderly subjects.
Keywords: Nobiletin; WMS‐R; elderly subject; memory function.
© 2021 The Authors. Food Science & Nutrition published by Wiley Periodicals LLC.
Conflict of interest statement
All authors declare that they have no conflicts of interest. All authors have approved the final draft of the manuscript.
Figures
Similar articles
-
Beneficial Effects of a Formulated Supplement of Ascidiacea (Halocynthia-roretzi)-derived Plasmalogen and Tuna-derived Elastin on Memory Function in Elderly Japanese Subjects; A Randomized, Double-blind, Placebo-controlled Study.J Oleo Sci. 2024 Oct 1;73(10):1319-1328. doi: 10.5650/jos.ess24128. Epub 2024 Sep 20. J Oleo Sci. 2024. PMID: 39313395 Clinical Trial.
-
Efficacy and Blood Plasmalogen Changes by Oral Administration of Plasmalogen in Patients with Mild Alzheimer's Disease and Mild Cognitive Impairment: A Multicenter, Randomized, Double-blind, Placebo-controlled Trial.EBioMedicine. 2017 Mar;17:199-205. doi: 10.1016/j.ebiom.2017.02.012. Epub 2017 Feb 24. EBioMedicine. 2017. PMID: 28259590 Free PMC article. Clinical Trial.
-
Perilla seed oil in combination with nobiletin-rich ponkan powder enhances cognitive function in healthy elderly Japanese individuals: a possible supplement for brain health in the elderly.Food Funct. 2022 Mar 7;13(5):2768-2781. doi: 10.1039/d1fo03508h. Food Funct. 2022. PMID: 35171190 Clinical Trial.
-
Anti-dementia Activity of Nobiletin, a Citrus Flavonoid: A Review of Animal Studies.Clin Psychopharmacol Neurosci. 2014 Aug;12(2):75-82. doi: 10.9758/cpn.2014.12.2.75. Epub 2014 Aug 12. Clin Psychopharmacol Neurosci. 2014. PMID: 25191498 Free PMC article. Review.
-
Differentiating Alzheimer's disease from Huntington's disease with the Wechsler Memory Scale-Revised.Clin Geriatr Med. 1989 Aug;5(3):611-32. Clin Geriatr Med. 1989. PMID: 2529959 Review.
Cited by
-
A Narrative Review of the Effects of Citrus Peels and Extracts on Human Brain Health and Metabolism.Nutrients. 2022 Apr 28;14(9):1847. doi: 10.3390/nu14091847. Nutrients. 2022. PMID: 35565814 Free PMC article. Review.
-
Cellular Internalization and Toxicity of Chitosan Nanoparticles Loaded with Nobiletin in Eukaryotic Cell Models (Saccharomyces cerevisiae and Candida albicans).Materials (Basel). 2024 Mar 27;17(7):1525. doi: 10.3390/ma17071525. Materials (Basel). 2024. PMID: 38612040 Free PMC article.
-
Nobiletin as an inducer of programmed cell death in cancer: a review.Apoptosis. 2022 Jun;27(5-6):297-310. doi: 10.1007/s10495-022-01721-4. Epub 2022 Mar 21. Apoptosis. 2022. PMID: 35312885 Review.
-
Enhancing circadian rhythms-the circadian MEGA bundle as novel approach to treat critical illness.Ann Transl Med. 2023 Jun 30;11(9):319. doi: 10.21037/atm-22-5127. Epub 2023 Jun 9. Ann Transl Med. 2023. PMID: 37404989 Free PMC article. Review.
-
Effectiveness and Safety of a Mixture of Nobiletin and Tangeretin in Nocturia Patients: A Randomized, Placebo-Controlled, Double-Blind, Crossover Study.J Clin Med. 2023 Apr 7;12(8):2757. doi: 10.3390/jcm12082757. J Clin Med. 2023. PMID: 37109094 Free PMC article.
References
-
- Braidy, N. , Behzad, S. , Habtemariam, S. , Ahmed, T. , Daglia, M. , Nabavi, S. M. , Sobarzo‐Sanchez, E. , & Nabavi, S. F. (2017). Neuroprotective effects of citrus fruit‐derived flavonoids, nobiletin and tangeretin in Alzheimer's and Parkinson's Disease. CNS & Neurological Disorders: Drug Targets, 16, 387–397. 10.2174/1871527316666170328113309 - DOI - PubMed
-
- Choi, Y. , Kim, Y. , Ham, H. , Park, Y. , Jeong, H.‐S. , & Lee, J. (2011). Nobiletin suppresses adipogenesis by regulating the expression of adipogenic transcription factors and the activation of AMP‐activated protein kinase (AMPK). Journal of Agriculture and Food Chemistry, 59, 12843–12849. 10.1021/jf2033208 - DOI - PubMed
-
- Chun, J. M. , Lee, A. R. , Kim, H. S. , Lee, A. Y. , Gu, G. J. , Moon, B. C. , & Kwon, B.‐I. (2018). Peucedanum japonicum extract attenuates allergic airway inflammation by inhibiting Th2 cell activation and production of pro‐inflammatory mediators. Journal of Ethnopharmacology, 211, 78–88. 10.1016/j.jep.2017.09.006 - DOI - PubMed
LinkOut - more resources
Full Text Sources