A Western diet with alcohol in drinking water recapitulates features of alcohol-associated liver disease in mice
- PMID: 34523155
- PMCID: PMC9006178
- DOI: 10.1111/acer.14700
A Western diet with alcohol in drinking water recapitulates features of alcohol-associated liver disease in mice
Abstract
Background: Mouse models of alcohol-associated liver disease vary greatly in their ease of implementation and the pathology they produce. Effects range from steatosis and mild inflammation with the Lieber-DeCarli liquid diet to severe inflammation, fibrosis, and pyroptosis seen with the Tsukamoto-French intragastric feeding model. Implementation of all of these models is limited by the labor-intensive nature of the protocols and the specialized skills necessary for successful intragastric feeding. We thus sought to develop a new model to reproduce features of alcohol-induced inflammation and fibrosis with minimal operational requirements.
Methods: Over a 16-week period, mice were fed ad libitum with a pelleted high-fat Western diet (WD; 40% calories from fat) and alcohol added to the drinking water. We found the optimal alcohol consumption to be that at which the alcohol concentration was 20% for 4 days and 10% for 3 days per week. Control mice received WD pellets with water alone.
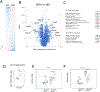
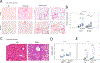
Results: Alcohol consumption was 18 to 20 g/kg/day in males and 20 to 22 g/kg/day in females. Mice in the alcohol groups developed elevated serum transaminase levels after 12 weeks in males and 10 weeks in females. At 16 weeks, both males and females developed liver inflammation, steatosis, and pericellular fibrosis. Control mice on WD without alcohol had mild steatosis only. Alcohol-fed mice showed reduced HNF4α mRNA and protein expression. HNF4α is a master regulator of hepatocyte differentiation, down-regulation of which is a known driver of hepatocellular failure in alcoholic hepatitis.
Conclusion: A simple-to-administer, 16-week WD alcohol model recapitulates the inflammatory, fibrotic, and gene expression aspects of human alcohol-associated steatohepatitis.
Keywords: HNF4α; fibrosis; gender differences; liver inflammation; steatohepatitis.
© 2021 by the Research Society on Alcoholism.
Conflict of interest statement
Figures





Similar articles
-
Fat-Specific Protein 27/CIDEC Promotes Development of Alcoholic Steatohepatitis in Mice and Humans.Gastroenterology. 2015 Oct;149(4):1030-41.e6. doi: 10.1053/j.gastro.2015.06.009. Epub 2015 Jun 20. Gastroenterology. 2015. PMID: 26099526 Free PMC article.
-
Mouse Model of Alcoholic Steatohepatitis.Methods Mol Biol. 2020;2164:145-157. doi: 10.1007/978-1-0716-0704-6_15. Methods Mol Biol. 2020. PMID: 32607891
-
MicroRNA expression profile in Lieber-DeCarli diet-induced alcoholic and methionine choline deficient diet-induced nonalcoholic steatohepatitis models in mice.Alcohol Clin Exp Res. 2009 Oct;33(10):1704-10. doi: 10.1111/j.1530-0277.2009.01007.x. Epub 2009 Jul 1. Alcohol Clin Exp Res. 2009. PMID: 19572984 Free PMC article.
-
Rodent Models of Alcoholic Liver Disease: Role of Binge Ethanol Administration.Biomolecules. 2018 Jan 13;8(1):3. doi: 10.3390/biom8010003. Biomolecules. 2018. PMID: 29342874 Free PMC article. Review.
-
Hepatocyte nuclear factor 4α in the pathogenesis of non-alcoholic fatty liver disease.Chin Med J (Engl). 2022 May 20;135(10):1172-1181. doi: 10.1097/CM9.0000000000002092. Chin Med J (Engl). 2022. PMID: 35191422 Free PMC article. Review.
Cited by
-
Extracellular matrix protein 1 binds to connective tissue growth factor against liver fibrosis and ductular reaction.Hepatol Commun. 2024 Oct 30;8(11):e0564. doi: 10.1097/HC9.0000000000000564. eCollection 2024 Nov 1. Hepatol Commun. 2024. PMID: 39470347 Free PMC article.
-
Protective role of 17β-estradiol in alcohol-associated liver fibrosis is mediated by suppression of integrin signaling.Hepatol Commun. 2024 May 3;8(5):e0428. doi: 10.1097/HC9.0000000000000428. eCollection 2024 May 1. Hepatol Commun. 2024. PMID: 38704651 Free PMC article.
-
New murine model of alcoholic hepatitis in obesity-induced metabolic-associated fatty liver disease.Exp Anim. 2023 Aug 7;72(3):389-401. doi: 10.1538/expanim.22-0160. Epub 2023 Apr 5. Exp Anim. 2023. PMID: 37019681 Free PMC article.
-
A novel experimental model of MetALD in male mice recapitulates key features of severe alcohol-associated hepatitis.Hepatol Commun. 2024 Jun 19;8(7):e0450. doi: 10.1097/HC9.0000000000000450. eCollection 2024 Jul 1. Hepatol Commun. 2024. PMID: 38896082 Free PMC article.
-
High-Fat Diet Augments the Effect of Alcohol on Skeletal Muscle Mitochondrial Dysfunction in Mice.Nutrients. 2022 Feb 28;14(5):1016. doi: 10.3390/nu14051016. Nutrients. 2022. PMID: 35267991 Free PMC article.
References
-
- ARGEMI J, LATASA MU, ATKINSON SR, BLOKHIN IO, MASSEY V, GUE JP, CABEZAS J, LOZANO JJ, VAN BOOVEN D, BELL A, CAO S, VERNETTI LA, ARAB JP, VENTURA-COTS M, EDMUNDS LR, FONDEVILLA C, STARKEL P, DUBUQUOY L, LOUVET A, ODENA G, GOMEZ JL, ARAGON T, ALTAMIRANO J, CABALLERIA J, JURCZAK MJ, TAYLOR DL, BERASAIN C, WAHLESTEDT C, MONGA SP, MORGAN MY, SANCHO-BRU P, MATHURIN P, FURUYA S, LACKNER C, RUSYN I, SHAH VH, THURSZ MR, MANN J, AVILA MA & BATALLER R 2019. Defective HNF4alpha-dependent gene expression as a driver of hepatocellular failure in alcoholic hepatitis. Nat Commun, 10, 3126. - PMC - PubMed
-
- BELKNAP JK, CRABBE JC & YOUNG ER 1993. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl), 112, 503–10. - PubMed
-
- BENEDÉ-UBIETO R, ESTÉVEZ-VÁZQUEZ O, GUO F, CHEN C, SINGH Y, NAKAYA HI, GÓMEZ DEL MORAL M, LAMAS-PAZ A, MORÁN L, LÓPEZ-ALCÁNTARA N, REISSING J, BRUNS T, AVILA MA, SANTAMARÍA E, MAZARIEGOS MS, WOITOK MM, HAAS U, ZHENG K, JUÁREZ I, MARTÍN-VILLA JM, ASENSIO I, VAQUERO J, PELIGROS MI, ARGEMI J, BATALLER R, AMPUERO J, ROMERO GÓMEZ M, TRAUTWEIN C, LIEDTKE C, BAÑARES R, CUBERO FJ & NEVZOROVA YA 2021. An Experimental DUAL Model of Advanced Liver Damage. Hepatol Commun, 5, 1051–1068. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

