Dynamic EMT: a multi-tool for tumor progression
- PMID: 34459003
- PMCID: PMC8441439
- DOI: 10.15252/embj.2021108647
Dynamic EMT: a multi-tool for tumor progression
Abstract
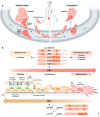
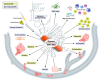
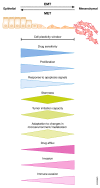
The process of epithelial-mesenchymal transition (EMT) is fundamental for embryonic morphogenesis. Cells undergoing it lose epithelial characteristics and integrity, acquire mesenchymal features, and become motile. In cancer, this program is hijacked to confer essential changes in morphology and motility that fuel invasion. In addition, EMT is increasingly understood to orchestrate a large variety of complementary cancer features, such as tumor cell stemness, tumorigenicity, resistance to therapy and adaptation to changes in the microenvironment. In this review, we summarize recent findings related to these various classical and non-classical functions, and introduce EMT as a true tumorigenic multi-tool, involved in many aspects of cancer. We suggest that therapeutic targeting of the EMT process will-if acknowledging these complexities-be a possibility to concurrently interfere with tumor progression on many levels.
Keywords: EMT; MET; SLUG; SNAIL; TWIST; ZEB1; ZEB2; cancer; cell plasticity; hybrid EMT; invasion; metastasis; partial EMT; signaling pathways; tumor stemness.
© 2021 The Authors. Published under the terms of the CC BY 4.0 license.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Mechanism of epithelial-mesenchymal transition in cancer and its regulation by natural compounds.Med Res Rev. 2023 Jul;43(4):1141-1200. doi: 10.1002/med.21948. Epub 2023 Mar 17. Med Res Rev. 2023. PMID: 36929669 Review.
-
EMT Transition States during Tumor Progression and Metastasis.Trends Cell Biol. 2019 Mar;29(3):212-226. doi: 10.1016/j.tcb.2018.12.001. Epub 2018 Dec 26. Trends Cell Biol. 2019. PMID: 30594349 Review.
-
Factors Determining Epithelial-Mesenchymal Transition in Cancer Progression.Int J Mol Sci. 2024 Aug 17;25(16):8972. doi: 10.3390/ijms25168972. Int J Mol Sci. 2024. PMID: 39201656 Free PMC article. Review.
-
Diverse pathways of epithelial mesenchymal transition related with cancer progression and metastasis and potential effects of endocrine disrupting chemicals on epithelial mesenchymal transition process.Mol Cell Endocrinol. 2017 Dec 5;457:103-113. doi: 10.1016/j.mce.2016.12.026. Epub 2016 Dec 29. Mol Cell Endocrinol. 2017. PMID: 28042023 Review.
-
MicroRNAs regulate the epithelial to mesenchymal transition (EMT) in cancer progression.Microrna. 2014;3(2):108-17. doi: 10.2174/2211536603666141010115102. Microrna. 2014. PMID: 25323025 Review.
Cited by
-
Elevated expression levels of the protein kinase DYRK1B induce mesenchymal features in A549 lung cancer cells.BMC Cancer. 2024 Oct 31;24(1):1341. doi: 10.1186/s12885-024-13057-0. BMC Cancer. 2024. PMID: 39482615 Free PMC article.
-
Integration of bioinformatics and cellular experiments unveils the role of SYT12 in gastric cancer.BMC Cancer. 2024 Oct 29;24(1):1331. doi: 10.1186/s12885-024-13077-w. BMC Cancer. 2024. PMID: 39472897 Free PMC article.
-
The complement C3a/C3aR pathway is associated with treatment resistance to gemcitabine-based neoadjuvant therapy in pancreatic cancer.Comput Struct Biotechnol J. 2024 Oct 5;23:3634-3650. doi: 10.1016/j.csbj.2024.09.032. eCollection 2024 Dec. Comput Struct Biotechnol J. 2024. PMID: 39469671 Free PMC article.
-
MicroRNAs in Hepatocellular Carcinoma: Insights into Regulatory Mechanisms, Clinical Significance, and Therapeutic Potential.Cancer Manag Res. 2024 Oct 19;16:1491-1507. doi: 10.2147/CMAR.S477698. eCollection 2024. Cancer Manag Res. 2024. PMID: 39450194 Free PMC article. Review.
-
Chromosome 8q24 amplification associated with human hepatocellular carcinoma predicts MYC/ZEB1/MIZ1 transcriptional regulation.Sci Rep. 2024 Oct 18;14(1):24488. doi: 10.1038/s41598-024-75219-1. Sci Rep. 2024. PMID: 39424877 Free PMC article.
References
-
- Ahmed N, Maines‐Bandiera S, Quinn MA, Unger WG, Dedhar S, Auersperg N (2006) Molecular pathways regulating EGF‐induced epithelio‐mesenchymal transition in human ovarian surface epithelium. Am J Physiol Cell Physiol 290: C1532–1542 - PubMed
-
- Akalay I, Janji B, Hasmim M, Noman MZ, André F, De Cremoux P, Bertheau P, Badoual C, Vielh P, Larsen AKet al (2013) Epithelial‐to‐mesenchymal transition and autophagy induction in breast carcinoma promote escape from T‐cell‐mediated lysis. Cancer Res 73: 2418–2427 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

