Functional Aging in Male C57BL/6J Mice Across the Life-Span: A Systematic Behavioral Analysis of Motor, Emotional, and Memory Function to Define an Aging Phenotype
- PMID: 34408644
- PMCID: PMC8365336
- DOI: 10.3389/fnagi.2021.697621
Functional Aging in Male C57BL/6J Mice Across the Life-Span: A Systematic Behavioral Analysis of Motor, Emotional, and Memory Function to Define an Aging Phenotype
Abstract
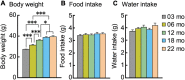
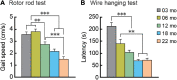
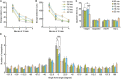
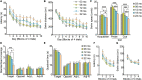
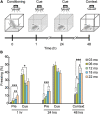
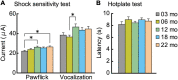
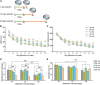
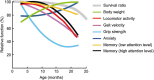
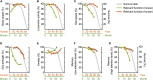
Aging is characterized generally by progressive and overall physiological decline of functions and is observed in all animals. A long line of evidence has established the laboratory mouse as the prime model of human aging. However, relatively little is known about the detailed behavioral and functional changes that occur across their lifespan, and how this maps onto the phenotype of human aging. To better understand age-related changes across the life-span, we characterized functional aging in male C57BL/6J mice of five different ages (3, 6, 12, 18, and 22 months of age) using a multi-domain behavioral test battery. Spatial memory and physical activities, including locomotor activity, gait velocity, and grip strength progressively declined with increasing age, although at different rates; anxiety-like behaviors increased with aging. Estimated age-related patterns showed that these functional alterations across ages are non-linear, and the patterns are unique for each behavioral trait. Physical function progressively declines, starting as early as 6 months of age in mice, while cognitive function begins to decline later, with considerable impairment present at 22 months of age. Importantly, functional aging of male C57BL/6J mouse starts at younger relative ages compared to when it starts in humans. Our study suggests that human-equivalent ages of mouse might be better determined on the basis of its functional capabilities.
Keywords: aging; animal models; anxiety; behavior rating scale; handgrip strength; inbred C57BL mice; locomotion; spatial memory.
Copyright © 2021 Yanai and Endo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
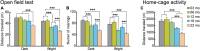
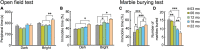
Similar articles
-
Age-related changes in behavior in C57BL/6J mice from young adulthood to middle age.Mol Brain. 2016 Jan 28;9:11. doi: 10.1186/s13041-016-0191-9. Mol Brain. 2016. PMID: 26822304 Free PMC article.
-
Age-related behavioral changes from young to old age in male mice of a C57BL/6J strain maintained under a genetic stability program.Neuropsychopharmacol Rep. 2019 Jun;39(2):100-118. doi: 10.1002/npr2.12052. Epub 2019 Feb 27. Neuropsychopharmacol Rep. 2019. PMID: 30816023 Free PMC article.
-
Impaired burrowing is the most prominent behavioral deficit of aging htau mice.Neuroscience. 2016 Aug 4;329:98-111. doi: 10.1016/j.neuroscience.2016.05.004. Epub 2016 May 7. Neuroscience. 2016. PMID: 27167086 Free PMC article.
-
Changes in Cognitive Function in Human Aging.In: Riddle DR, editor. Brain Aging: Models, Methods, and Mechanisms. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 1. In: Riddle DR, editor. Brain Aging: Models, Methods, and Mechanisms. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 1. PMID: 21204355 Free Books & Documents. Review.
-
The aged mouse as a model of cognitive decline with special emphasis on studies in NMRI mice.Behav Brain Res. 1993 Nov 30;57(2):163-73. doi: 10.1016/0166-4328(93)90132-a. Behav Brain Res. 1993. PMID: 8117421 Review.
Cited by
-
Efficacy of a Combination Therapy with Laronidase and Genistein in Treating Mucopolysaccharidosis Type I in a Mouse Model.Int J Mol Sci. 2024 Feb 17;25(4):2371. doi: 10.3390/ijms25042371. Int J Mol Sci. 2024. PMID: 38397051 Free PMC article.
-
Longitudinal home-cage automated assessment of climbing behavior shows sexual dimorphism and aging-related decrease in C57BL/6J healthy mice and allows early detection of motor impairment in the N171-82Q mouse model of Huntington's disease.Front Behav Neurosci. 2023 Mar 22;17:1148172. doi: 10.3389/fnbeh.2023.1148172. eCollection 2023. Front Behav Neurosci. 2023. PMID: 37035623 Free PMC article.
-
Nature of epigenetic aging from a single-cell perspective.Nat Aging. 2024 Jun;4(6):854-870. doi: 10.1038/s43587-024-00616-0. Epub 2024 May 9. Nat Aging. 2024. PMID: 38724733
-
Long-lasting geroprotection from brief rapamycin treatment in early adulthood by persistently increased intestinal autophagy.Nat Aging. 2022 Sep;2(9):824-836. doi: 10.1038/s43587-022-00278-w. Epub 2022 Aug 29. Nat Aging. 2022. PMID: 37118497 Free PMC article.
-
Reduced insulin signaling in neurons induces sex-specific health benefits.Sci Adv. 2023 Feb 22;9(8):eade8137. doi: 10.1126/sciadv.ade8137. Epub 2023 Feb 22. Sci Adv. 2023. PMID: 36812323 Free PMC article.
References
-
- Bach M. E., Barad M., Son H., Zhuo M., Lu Y. F., Shih R., et al. (1999). Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc. Natl. Acad. Sci. U. S. A. 96 5280–5285. 10.1371/10.1073/pnas.96.9.5280 - DOI - PMC - PubMed
-
- Benice T. S., Rizk A., Kohama S., Pfankuch T., Raber J. (2006). Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience 137 413–423. 10.1016/j.neuroscience.2005.08.029 - DOI - PubMed
LinkOut - more resources
Full Text Sources

