Subcutaneous REGEN-COV Antibody Combination to Prevent Covid-19
- PMID: 34347950
- PMCID: PMC8362593
- DOI: 10.1056/NEJMoa2109682
Subcutaneous REGEN-COV Antibody Combination to Prevent Covid-19
Abstract
Background: REGEN-COV (previously known as REGN-COV2), a combination of the monoclonal antibodies casirivimab and imdevimab, has been shown to markedly reduce the risk of hospitalization or death among high-risk persons with coronavirus disease 2019 (Covid-19). Whether subcutaneous REGEN-COV prevents severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and subsequent Covid-19 in persons at high risk for infection because of household exposure to a person with SARS-CoV-2 infection is unknown.
Methods: We randomly assigned, in a 1:1 ratio, participants (≥12 years of age) who were enrolled within 96 hours after a household contact received a diagnosis of SARS-CoV-2 infection to receive a total dose of 1200 mg of REGEN-COV or matching placebo administered by means of subcutaneous injection. At the time of randomization, participants were stratified according to the results of the local diagnostic assay for SARS-CoV-2 and according to age. The primary efficacy end point was the development of symptomatic SARS-CoV-2 infection through day 28 in participants who did not have SARS-CoV-2 infection (as measured by reverse-transcriptase-quantitative polymerase-chain-reaction assay) or previous immunity (seronegativity).
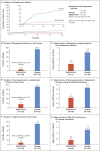
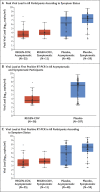
Results: Symptomatic SARS-CoV-2 infection developed in 11 of 753 participants in the REGEN-COV group (1.5%) and in 59 of 752 participants in the placebo group (7.8%) (relative risk reduction [1 minus the relative risk], 81.4%; P<0.001). In weeks 2 to 4, a total of 2 of 753 participants in the REGEN-COV group (0.3%) and 27 of 752 participants in the placebo group (3.6%) had symptomatic SARS-CoV-2 infection (relative risk reduction, 92.6%). REGEN-COV also prevented symptomatic and asymptomatic infections overall (relative risk reduction, 66.4%). Among symptomatic infected participants, the median time to resolution of symptoms was 2 weeks shorter with REGEN-COV than with placebo (1.2 weeks and 3.2 weeks, respectively), and the duration of a high viral load (>104 copies per milliliter) was shorter (0.4 weeks and 1.3 weeks, respectively). No dose-limiting toxic effects of REGEN-COV were noted.
Conclusions: Subcutaneous REGEN-COV prevented symptomatic Covid-19 and asymptomatic SARS-CoV-2 infection in previously uninfected household contacts of infected persons. Among the participants who became infected, REGEN-COV reduced the duration of symptomatic disease and the duration of a high viral load. (Funded by Regeneron Pharmaceuticals and others; ClinicalTrials.gov number, NCT04452318.).
Copyright © 2021 Massachusetts Medical Society.
Figures
Update of
-
Subcutaneous REGEN-COV Antibody Combination for Covid-19 Prevention.medRxiv [Preprint]. 2021 Jun 17:2021.06.14.21258567. doi: 10.1101/2021.06.14.21258567. medRxiv. 2021. Update in: N Engl J Med. 2021 Sep 23;385(13):1184-1195. doi: 10.1056/NEJMoa2109682. PMID: 34159344 Free PMC article. Updated. Preprint.
Comment in
-
Subcutaneous REGEN-COV Antibody Combination to Prevent Covid-19.N Engl J Med. 2021 Nov 11;385(20):e70. doi: 10.1056/NEJMc2113862. Epub 2021 Oct 6. N Engl J Med. 2021. PMID: 34614318 No abstract available.
-
In uninfected household contacts of patients with COVID-19, REGEN-COV reduced symptomatic COVID-19 at 28 d.Ann Intern Med. 2022 Jan;175(1):JC5. doi: 10.7326/J21-0008. Epub 2022 Jan 4. Ann Intern Med. 2022. PMID: 34978854
Similar articles
-
Effect of Subcutaneous Casirivimab and Imdevimab Antibody Combination vs Placebo on Development of Symptomatic COVID-19 in Early Asymptomatic SARS-CoV-2 Infection: A Randomized Clinical Trial.JAMA. 2022 Feb 1;327(5):432-441. doi: 10.1001/jama.2021.24939. JAMA. 2022. PMID: 35029629 Free PMC article. Clinical Trial.
-
REGEN-COV Antibody Combination and Outcomes in Outpatients with Covid-19.N Engl J Med. 2021 Dec 2;385(23):e81. doi: 10.1056/NEJMoa2108163. Epub 2021 Sep 29. N Engl J Med. 2021. PMID: 34587383 Free PMC article. Clinical Trial.
-
Subcutaneous REGEN-COV Antibody Combination in Early Asymptomatic SARS-CoV-2 Infection: A Randomized Clinical Trial.medRxiv [Preprint]. 2021 Sep 18:2021.06.14.21258569. doi: 10.1101/2021.06.14.21258569. medRxiv. 2021. Update in: JAMA. 2022 Feb 1;327(5):432-441. doi: 10.1001/jama.2021.24939. PMID: 34159343 Free PMC article. Updated. Preprint.
-
SARS-CoV-2-neutralising monoclonal antibodies to prevent COVID-19.Cochrane Database Syst Rev. 2022 Jun 17;6(6):CD014945. doi: 10.1002/14651858.CD014945.pub2. Cochrane Database Syst Rev. 2022. PMID: 35713300 Free PMC article. Review.
-
Casirivimab/Imdevimab: First Approval.Drugs. 2021 Nov;81(17):2047-2055. doi: 10.1007/s40265-021-01620-z. Drugs. 2021. PMID: 34716907 Free PMC article. Review.
Cited by
-
Therapeutic potential of mesenchymal stem cell-derived extracellular vesicles in SARS-CoV-2 and H1N1 influenza-induced acute lung injury.J Extracell Vesicles. 2024 Sep;13(9):e12495. doi: 10.1002/jev2.12495. J Extracell Vesicles. 2024. PMID: 39254228 Free PMC article.
-
Managing and treating COVID-19 in patients with hematological malignancies: a narrative review and expert insights.Clin Exp Med. 2024 Jun 4;24(1):119. doi: 10.1007/s10238-024-01381-5. Clin Exp Med. 2024. PMID: 38833206 Free PMC article. Review.
-
SARS-CoV-2 resistance to monoclonal antibodies and small-molecule drugs.Cell Chem Biol. 2024 Apr 18;31(4):632-657. doi: 10.1016/j.chembiol.2024.03.008. Cell Chem Biol. 2024. PMID: 38640902 Review.
-
Utilization of SARS-COV-2 positive donors and recipients for liver transplantation in the pandemic era - An evidence-based review.J Liver Transpl. 2022 Jul-Sep;7:100081. doi: 10.1016/j.liver.2022.100081. Epub 2022 Mar 11. J Liver Transpl. 2022. PMID: 38620745 Free PMC article.
-
Concordance between SARS-CoV-2 index individuals and their household contacts on index individual COVID-19 transmission cofactors: a comparison of self-reported and contact-reported information.BMC Public Health. 2024 Apr 2;24(1):950. doi: 10.1186/s12889-024-18371-7. BMC Public Health. 2024. PMID: 38566051 Free PMC article. Clinical Trial.
References
-
- World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19 — 11 March 2020. 2020. (https://www.who.int/director-general/speeches/detail/who-director-genera...).
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
