Cepharanthine sensitizes human triple negative breast cancer cells to chemotherapeutic agent epirubicin via inducing cofilin oxidation-mediated mitochondrial fission and apoptosis
- PMID: 34294886
- PMCID: PMC8724299
- DOI: 10.1038/s41401-021-00715-3
Cepharanthine sensitizes human triple negative breast cancer cells to chemotherapeutic agent epirubicin via inducing cofilin oxidation-mediated mitochondrial fission and apoptosis
Abstract
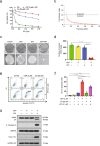
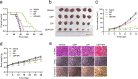
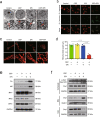
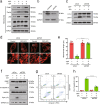
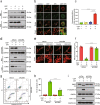
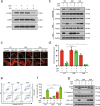
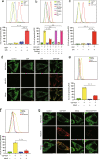
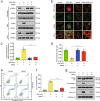
Inhibition of autophagy has been accepted as a promising therapeutic strategy in cancer, but its clinical application is hindered by lack of effective and specific autophagy inhibitors. We previously identified cepharanthine (CEP) as a novel autophagy inhibitor, which inhibited autophagy/mitophagy through blockage of autophagosome-lysosome fusion in human breast cancer cells. In this study we investigated whether and how inhibition of autophagy/mitophagy by cepharanthine affected the efficacy of chemotherapeutic agent epirubicin in triple negative breast cancer (TNBC) cells in vitro and in vivo. In human breast cancer MDA-MB-231 and BT549 cells, application of CEP (2 μM) greatly enhanced cepharanthine-induced inhibition on cell viability and colony formation. CEP interacted with epirubicin synergistically to induce apoptosis in TNBC cells via the mitochondrial pathway. We demonstrated that co-administration of CEP and epirubicin induced mitochondrial fission in MDA-MB-231 cells, and the production of mitochondrial superoxide was correlated with mitochondrial fission and apoptosis induced by the combination. Moreover, we revealed that co-administration of CEP and epirubicin markedly increased the generation of mitochondrial superoxide, resulting in oxidation of the actin-remodeling protein cofilin, which promoted formation of an intramolecular disulfide bridge between Cys39 and Cys80 as well as Ser3 dephosphorylation, leading to mitochondria translocation of cofilin, thus causing mitochondrial fission and apoptosis. Finally, in mice bearing MDA-MB-231 cell xenografts, co-administration of CEP (12 mg/kg, ip, once every other day for 36 days) greatly enhanced the therapeutic efficacy of epirubicin (2 mg/kg) as compared with administration of either drug alone. Taken together, our results implicate that a combination of cepharanthine with chemotherapeutic agents could represent a novel therapeutic strategy for the treatment of breast cancer.
Keywords: apoptosis; cepharanthine; cofilin; epirubicin; mitochondrial fission; mitochondrial superoxide; oxidative stress; triple negative breast cancer.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interest.
Figures

Similar articles
-
Research progress on pharmacological effects and mechanisms of cepharanthine and its derivatives.Naunyn Schmiedebergs Arch Pharmacol. 2023 Nov;396(11):2843-2860. doi: 10.1007/s00210-023-02537-y. Epub 2023 Jun 20. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 37338575 Review.
-
ROS-mediated activation and mitochondrial translocation of CaMKII contributes to Drp1-dependent mitochondrial fission and apoptosis in triple-negative breast cancer cells by isorhamnetin and chloroquine.J Exp Clin Cancer Res. 2019 May 28;38(1):225. doi: 10.1186/s13046-019-1201-4. J Exp Clin Cancer Res. 2019. PMID: 31138329 Free PMC article.
-
Downregulation of MYO1C mediated by cepharanthine inhibits autophagosome-lysosome fusion through blockade of the F-actin network.J Exp Clin Cancer Res. 2019 Nov 7;38(1):457. doi: 10.1186/s13046-019-1449-8. J Exp Clin Cancer Res. 2019. PMID: 31699152 Free PMC article.
-
ROCK1 activation-mediated mitochondrial translocation of Drp1 and cofilin are required for arnidiol-induced mitochondrial fission and apoptosis.J Exp Clin Cancer Res. 2020 Feb 19;39(1):37. doi: 10.1186/s13046-020-01545-7. J Exp Clin Cancer Res. 2020. PMID: 32075676 Free PMC article.
-
A mechanistic updated overview on Cepharanthine as potential anticancer agent.Biomed Pharmacother. 2023 Sep;165:115107. doi: 10.1016/j.biopha.2023.115107. Epub 2023 Jul 7. Biomed Pharmacother. 2023. PMID: 37423171 Review.
Cited by
-
DSTYK Enhances Chemoresistance in Triple-Negative Breast Cancer Cells.Cells. 2021 Dec 29;11(1):97. doi: 10.3390/cells11010097. Cells. 2021. PMID: 35011659 Free PMC article.
-
Roles of mitochondrial fusion and fission in breast cancer progression: a systematic review.World J Surg Oncol. 2022 Oct 3;20(1):331. doi: 10.1186/s12957-022-02799-5. World J Surg Oncol. 2022. PMID: 36192752 Free PMC article. Review.
-
Significance of platelet adhesion-related genes in colon cancer based on non-negative matrix factorization-based clustering algorithm.Digit Health. 2023 Sep 26;9:20552076231203902. doi: 10.1177/20552076231203902. eCollection 2023 Jan-Dec. Digit Health. 2023. PMID: 37766908 Free PMC article.
-
Research progress on pharmacological effects and mechanisms of cepharanthine and its derivatives.Naunyn Schmiedebergs Arch Pharmacol. 2023 Nov;396(11):2843-2860. doi: 10.1007/s00210-023-02537-y. Epub 2023 Jun 20. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 37338575 Review.
-
Promoting Apoptosis, a Promising Way to Treat Breast Cancer With Natural Products: A Comprehensive Review.Front Pharmacol. 2022 Jan 28;12:801662. doi: 10.3389/fphar.2021.801662. eCollection 2021. Front Pharmacol. 2022. PMID: 35153757 Free PMC article. Review.
References
-
- Fendt SM, Lunt SY. Dynamic ROS regulation by TIGAR: balancing anti-cancer and pro-metastasis effects. Cancer Cell. 2020;37:141–2. - PubMed
-
- Chen Y, Gibson SB. Is mitochondrial generation of reactive oxygen species a trigger for autophagy? Autophagy. 2008;4:246–8. - PubMed
-
- Ishii N, Ishii T, Hartman PS. The role of the electron transport SDHC gene on lifespan and cancer. Mitochondrion. 2007;7:24–28. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

