MicroRNA-26a-3p rescues depression-like behaviors in male rats via preventing hippocampal neuronal anomalies
- PMID: 34228643
- PMCID: PMC8363293
- DOI: 10.1172/JCI148853
MicroRNA-26a-3p rescues depression-like behaviors in male rats via preventing hippocampal neuronal anomalies
Abstract
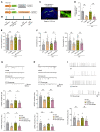
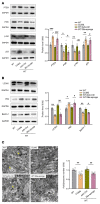
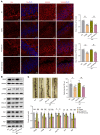
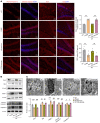
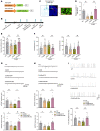
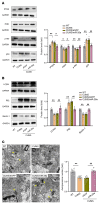
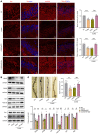
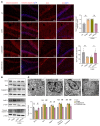
Depression is a neuropsychiatric disease associated with neuronal anomalies within specific brain regions. In the present study, we screened microRNA (miRNA) expression profiles in the dentate gyrus (DG) of the hippocampus and found that miR-26a-3p was markedly downregulated in a rat model of depression, whereas upregulation of miR-26a-3p within DG regions rescued the neuronal deterioration and depression-like phenotypes resulting from stress exposure, effects that appear to be mediated by the PTEN pathway. The knockdown of miR-26a-3p in DG regions of normal control rats induced depression-like behaviors, effects that were accompanied by activation of the PTEN/PI3K/Akt signaling pathway and neuronal deterioration via suppression of autophagy, impairments in synaptic plasticity, and promotion of neuronal apoptosis. In conclusion, these results suggest that miR-26a-3p deficits within the hippocampal DG mediated the neuronal anomalies contributing to the display of depression-like behaviors. This miRNA may serve as a potential therapeutic target for the treatment of depression.
Keywords: Behavior; Depression; Neuroscience; Synapses.
Conflict of interest statement
Figures


Similar articles
-
Downregulation of lncRNA GAS5 Alleviates Hippocampal Neuronal Damage in Mice with Depression-Like Behaviors Via Modulation of MicroRNA-26a/EGR1 Axis.J Stroke Cerebrovasc Dis. 2021 Mar;30(3):105550. doi: 10.1016/j.jstrokecerebrovasdis.2020.105550. Epub 2020 Dec 18. J Stroke Cerebrovasc Dis. 2021. PMID: 33341564
-
Downregulation of microRNA-200c-3p reduces damage of hippocampal neurons in epileptic rats by upregulating expression of RECK and inactivating the AKT signaling pathway.Chem Biol Interact. 2019 Jul 1;307:223-233. doi: 10.1016/j.cbi.2019.04.027. Epub 2019 Apr 21. Chem Biol Interact. 2019. PMID: 31018114
-
The targeted regulation of miR-26a on PTEN-PI3K/AKT signaling pathway in myocardial fibrosis after myocardial infarction.Eur Rev Med Pharmacol Sci. 2018 Jan;22(2):523-531. doi: 10.26355/eurrev_201801_14205. Eur Rev Med Pharmacol Sci. 2018. Retraction in: Eur Rev Med Pharmacol Sci. 2020 Sep;24(18):9235. doi: 10.26355/eurrev_202009_22989. PMID: 29424913 Retracted.
-
Hippocampal microRNA-26a-3p deficit contributes to neuroinflammation and behavioral disorders via p38 MAPK signaling pathway in rats.J Neuroinflammation. 2022 Nov 24;19(1):283. doi: 10.1186/s12974-022-02645-1. J Neuroinflammation. 2022. PMID: 36434679 Free PMC article.
-
Synaptic plasticity and depression: the role of miRNAs dysregulation.Mol Biol Rep. 2022 Oct;49(10):9759-9765. doi: 10.1007/s11033-022-07461-7. Epub 2022 Apr 20. Mol Biol Rep. 2022. PMID: 35441941 Review.
Cited by
-
Evidence for the Contribution of the miR-206/BDNF Pathway in the Pathophysiology of Depression.Int J Neuropsychopharmacol. 2024 Oct 1;27(10):pyae039. doi: 10.1093/ijnp/pyae039. Int J Neuropsychopharmacol. 2024. PMID: 39219169 Free PMC article. Review.
-
MicroRNA 3' ends shorten during adolescent brain maturation.Front Mol Neurosci. 2023 Apr 14;16:1168695. doi: 10.3389/fnmol.2023.1168695. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37122627 Free PMC article.
-
Stress-induced reduction of Na+/H+ exchanger isoform 1 promotes maladaptation of neuroplasticity and exacerbates depressive behaviors.Sci Adv. 2022 Nov 11;8(45):eadd7063. doi: 10.1126/sciadv.add7063. Epub 2022 Nov 11. Sci Adv. 2022. PMID: 36367929 Free PMC article.
-
Small Differences and Big Changes: The Many Variables of MicroRNA Expression and Function in the Brain.J Neurosci. 2024 Aug 7;44(32):e0365242024. doi: 10.1523/JNEUROSCI.0365-24.2024. J Neurosci. 2024. PMID: 39111834 Free PMC article. Review.
-
miR-204-5p Plays a Critical Role in the Pathogenesis of Depression and Anti-depression Action of Venlafaxine in the Hippocampus of Mice.Curr Med Chem. 2024;31(22):3412-3425. doi: 10.2174/0929867330666230623163315. Curr Med Chem. 2024. PMID: 37357509
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

